IJCRR - 9(16), August, 2017
Pages: 27-35
Print Article
Download XML Download PDF
Modulation of Antioxidant Status of Erythrocyte of In Vivo Heat Exposed Rats Fed on Altered Dietary Protein Level
Author: Sudhamay Ghosh, Ajay K. Chatterjee
Category: Healthcare
Abstract:This study was carried out to determine the effect of altered dietary protein level on the antioxidant status of erythrocytes of in vivo heat exposed rats. Experiments were performed on adult (80-90 days old) male Wister strain rats, divided in six groups of five animals each. Animals were exposed to 43�1 �C, ambient humidity conditions for 3 h daily for 15 days in a well maintained climatic chamber. Increased TBARS productions were found when measured in packed erythrocytes and erythrocyte membranes in both the rats fed on 18% or 6% protein diet after acute and chronic heat exposure. Supplementation of ascorbic acid to the rats fed on 18% or 6% protein diet was found to reduce the TBARS production remarkably in packed erythrocytes while it further raised the TBARS production in erythrocyte membrane in response to chronic and acute heat exposure. The liberation of alanine increased in 18% protein-fed as well as 6% protein-fed rats following chronic and acute exposure to heat. Decreased GSH content and increased membrane total thiol (-SH) content of erythrocytes were observed in both 18% protein-fed and 6% protein-fed rats. Prior supplementation of ascorbic acid was found to restore partially the GSH content of erythrocyte and to potentiate the increase in membrane total thiol (–SH) content in both adequately protein-fed and protein-restricted groups of rats.
Keywords: Ascorbic acid, Erythrocyte, Dietary protein, Heat stress, Rat
DOI: 10.7324/IJCRR.2017.9166
Full Text:
Introduction
Red cells are always under high oxygen pressure and extremely susceptible to peroxidation and the conditions that favour peroxidation, are seemingly optimal in red cells [1]. Due to their function in carrying oxygen and their high iron content, erythrocytes are constantly exposed to oxidative stress [2]. Bernabucci et al. suggested that erythrocytes are an appropriate and sensitive model to study the oxidative status of transition dairy cows exposed to hot environment [3]. On the other hand, studies demonstrated that heat stress induces oxidative stress in the body [4, 5,6]. It has been demonstrated that heat stress is one of the most important stressors in the hot regions of the world(Altan, 2003).It results the generation of free radicals and other reactive oxygen species(ROS) (Chihuailaf et al., 2002) which leads to cellular and tissue damage (Tkaczyk andVizek, 2007). Free radicals and ROS have been demonstrated to have adverse effects on erythrocytes [7,8,9,10].Oxidative stress has been implicated with increased protein degradation through several mechanisms, including the activation of proteases and increasing the expression of genes involved in autophagy and proteolysis [11, 12,13]. ROS have been shown to impair protein synthesis by preventing mRNA translation [14, 15, 16, 17,18].Acute heat-stressed broiler chickens had a 2-fold increase in malondialdehyde, a marker of lipid peroxidation, in skeletal muscle [19]. End-product of lipid peroxidation, acts as a heat shock protein (hsp) inducer [20]. Membrane cholesterol contents modify the protective power of flavonoids against oxidative stress in erythrocytes [21].The antioxidant power of flavonoids, in turn depend on the depletion or incorporation of cholesterol in the erythrocyte membrane [22]. Ascorbic Acid is an outstanding antioxidant found in the human blood plasma [23] and it stabilizes free radicals and terminates free radical induced lipid peroxidation of cytochromes, thereby maintaining the structural integrity of cells [10,24].Further, it is known that ascorbic acid along with electrolyte supplementation was found to ameliorate the heat stress in buffaloes and plays a key role in the modulation of glutathione oxidase-reeducates system [25].It is concluded that specific cytosolic proteins are trans located to the membrane in human erythrocytes exposed to heat stress and they may play a novel role as erythrocyte membrane protectors under stress by stabilizing the membrane skeleton through their interactions with skeletal proteins(Sharma S, Zingde SM, Gokhale SM., 2013)[26]. Again, it is reported that the responses to heat stress are not only dependent on denatured proteins. The membrane lipid changes and the associated production of signalling metabolites clearly have a pivotal function to modify the heat stress response [27].In addition, dietary protein level is also known to have profound influence on antioxidant system and membrane composition and stability[28]. Nutritional modifications may help animals to prevent nutrient deficiencies that result from heat stress. Lower food intake during hot weather reduces nutrients available for absorption, and absorbed nutrients are used less efficiently [28].
Accordingly, the aim of the present investigation is to study the effect in vivo of heat stress on biochemical changes of erythrocyte in terms of antioxidant status, lipid peroxidation and glutathione metabolism on dietary protein adequacy and inadequacy. It is also intended to note whether ascorbic acid supplementation has any protective effects against chronic and acutely heat-induced membrane changes.
Materials and methods
Chemicals
Bovine serum albumin (BSA), 5, 5/-dithiobistrinitrobenzoic acid (DTNB), Folin-Ciocalteau reagent, tris, disodium ethylene tetra acetate (EDTA), reduced glutathione (GSH), were purchased from SRL, Mumbai, India. 5-Sulphosalicylic acid dihydrate and 2-thiobarbituric acid (TBA) were purchased from Spectrochem, Mumbai, India. Other chemicals used throughout the investigation were of analytical grade.
Animals and diets
Male growing rats of Wistar strain weighing 100-120g were used for the present study. The animals were kept in a well- ventilated room with 12 hrs. day - light cycle. The animals were accustomed with this condition for 7 days with adequate amount of food containing protein (casein)18%, carbohydrate(amylum) 71%, fat(groundnut oil) 7%, salt mixture 4% and adequate amount of vitamins mixture as reported elsewhere [29]. The composition of the salt mixture used was as described by Hawk and Oser [30].Then the animals were divided into four groups of equal average body weight. The animals of half of the groups were continued with diet containing 18% protein while those of the remaining groups were maintained on the diet containing 6% protein, and 83% carbohydrate. The 18% protein was used as it was considered as an adequate (normal) dietary protein level which was used on earlier occasions [29]. The 6% protein was used as an inadequate dietary protein level (protein inadequacy) to study the influence of dietary protein inadequacy. This experiment was approved by the guidelines of Institutional Animal Ethics Committee of department of Physiology, University of Calcutta.
Heat Exposure
After maintaining for three weeks on experimental diets, rats of experimental groups were exposed to heat stress. From one week before the onset of heat exposure, body weight, food intake and rectal temperature had been recorded on every alternate day till the termination of the heat-exposure period. Rats were exposed to heat stress in a well maintained climatic chamber.
Ascorbic acid was supplemented to the rats at a dose of 20mg per 100g of body weight intraperitoneally from one week before the beginning of heat exposure to increase the antioxidant reserves of the rats, and supplementation was continued till the day before their sacrifice. Effective thermal stress was determined by varying the duration of heat exposure and keeping the exposure temperature constant and vice versa. In both conditions rectal temperature of each rat was recorded at regular time intervals and also after the termination of heat-exposure in each day to know the pattern and degree of heat stress imposed on the rats. Following these approaches temperature of 43 10C with 2 hrs. duration per day for 15 successive days and 43
10C with 2 hrs. duration per day for 15 successive days and 43 10C temperature with 3 hrs. duration in one day were considered optimum to produce the effect of chronic and acute heat exposure, respectively. Rats were exposed to heat between 2 and 6 p.m. in each day to avoid the diurnal variation of temperature. To maintain the uniformity in the heat stress induced, no experiment was performed in the months of summer (April to June) and winter (December to February). The entire study was carried out with several sets of experiments involving different groups of rats and keeping all the above conditions identical.
10C temperature with 3 hrs. duration in one day were considered optimum to produce the effect of chronic and acute heat exposure, respectively. Rats were exposed to heat between 2 and 6 p.m. in each day to avoid the diurnal variation of temperature. To maintain the uniformity in the heat stress induced, no experiment was performed in the months of summer (April to June) and winter (December to February). The entire study was carried out with several sets of experiments involving different groups of rats and keeping all the above conditions identical.
Tissue Collection
At the end of experimental period rats were kept fasting for 18 hrs. and then sacrificed by cervical dislocation. Blood was collected immediately from the hepatic vein with a heparinized syringe and kept in polypropylene vials at 40C, taking proper care to prevent any chance of haemolysis. To obtain erythrocytes, heparinized blood was centrifuged (1000xg at 40C for 10 mins.). Plasma was collected and stored in deep freeze. The buffy layer was removed completely by aspiration. The erythrocytes were washed three times with 20mM Tris-buffered-saline solution. The washed and packed erythrocytes were used for the preparation of ghost membrane.
Preparation of Erythrocyte Membrane
Fresh blood obtained from the rat was used to prepare ghost membrane following the procedure as developed by Marchesi and Pallade [31]. The packed red cells were washed thrice in 130mM NaCl and 20mM Tris-HCl (pH 7.4) mixture and recovered by centrifugation at 2500 r.p.m. for 15mins. Saline-washed red cells were lysed in 5mM Tris-HCl (pH 7.4) buffer containing 1mM EDTA and kept at 40C for 15 mins. Thirty nine volumes of haemolysing fluid were added to 1 volume of packed red cell, followed by centrifugation at 25,000g for 30 mins. at 40C. The supernatant was discarded and the membrane settled down was resuspended in the same medium and centrifuged again. The same procedure was repeated for 3 to 4 times until the membrane became milky white. Finally, the membrane was suspended in 50mM Tris-HCl buffer (pH 7.4) containing 1mM EDTA and kept frozen. All operations were carried out in cold.
Estimation of Membrane Protein
The protein was estimated by modified Lowry method [32]. The absorbance was read at 500nm and the protein content was calculated using a standard curve prepared with aliquots of BSA-solution having known concentration.
Statistical analysis
The data obtained from each experiment described above (N<30) were subjected to statistical analysis. The level of significance of the observed changes between the control and treated groups of animals was calculated according to two-tail student's 't'-test and the probability of chance of occurrence (p) was determined according to the Table (Level of significance for two-tail 't'-test) of Fisher and Yates [33]. The data were expressed as means  SEM. Differences were considered significant at p<0.05.
SEM. Differences were considered significant at p<0.05.
Results
Ascorbic Acid Status of Blood
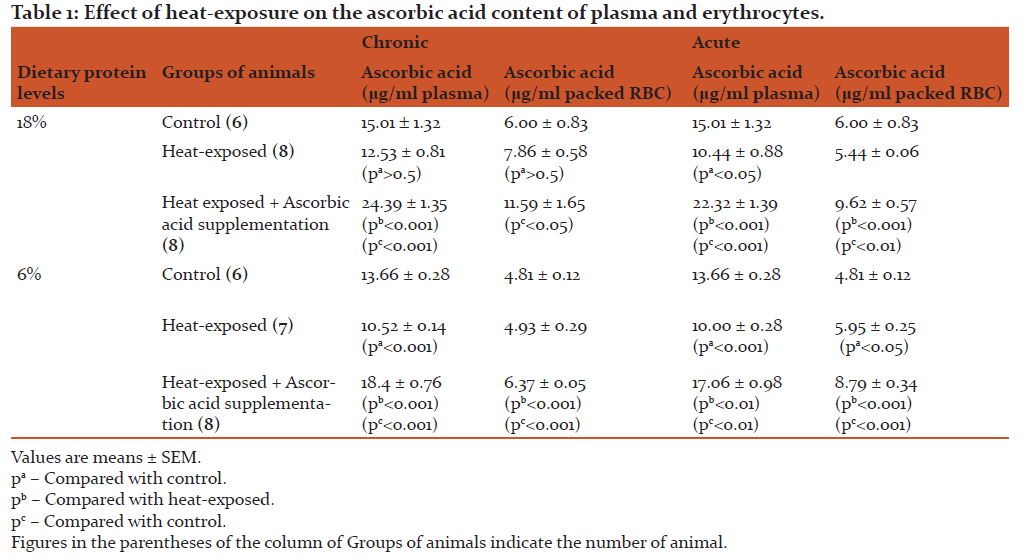
The results presented in Table 1 reveal the decrease in plasma ascorbic acid level following acute heat exposure in 18% protein-fed rats as well as 6% protein-fed rats. The decrease in plasma ascorbic acid level was also observed after chronic heat exposure in 6% protein-fed rats. But, when the ascorbic acid content was estimated in erythrocytes it was found to be diminished after acute heat exposure in protein-restricted rats only.
Erythrocyte Thiobarbituric Acid Reactive Substances (TBARS)
The results presented in Table 2A demonstrate that the rats receiving an 18% or 6% protein diet showed after chronic exposure to heat increased TBARS production when measured in packed erythrocytes and erythrocyte membranes. Prior supplementation of ascorbic acid to the rats fed on 18% or 6% protein diet was found to reduce the TBARS production remarkably in packed erythrocytes while it further raised the TBARS production in erythrocyte membrane in response to chronic heat exposure.
Acute heat exposure was also found to increase the TBARS production in packed erythrocytes as well as in erythrocyte membrane (Table 2B). The Tables 2A and 2B also indicate that the formation of TBARS in erythrocyte membrane of rats fed on an 18% or a 6% protein diet was quite higher following acute heat exposure than chronic exposure to heat. Prior supplementation of ascorbic acid restored partially the production of TBARS in packed erythrocyte but elevated its production in erythrocyte membrane of rats fed on an 18% as well as a 6% protein diet.
Formation of Conjugated Diene and Liberation of Alanine in Erythrocyte Membrane
The results presented in Table 2A reveal that the formation of conjugated diene increased in erythrocyte membrane following chronic exposure to heat. Further, the formation of conjugated diene appeared quite higher in protein-restricted rats than in adequately protein-fed rats. Alanine was also liberated in higher amount in both 18% protein-fed and 6% protein-fed groups of rats following chronic exposure to heat (Table 2A).
Acute heat exposure showed no effect on the formation of conjugated diene in 18% protein-fed rats but it elevated the formation of conjugated diene in rats fed a 6% protein diet (Table 2B). The liberation of alanine increased in 18% protein-fed as well as 6% protein-fed rats following acute exposure to heat (Table 2B).
Reduced Glutathione (GSH) and Membrane-thiol (-SH) Level of Erythrocytes
Table 3A shows that following chronic exposure to heat, the GSH content of erythrocyte was reduced in both 18% protein-fed and 6% protein-fed rats. The results also demonstrate that the extent of decrease in GSH content of erythrocyte in protein-restricted group of rats following chronic exposure to heat was further accentuated.
Prior supplementation of ascorbic acid restored partially the GSH content of erythrocyte in both adequately protein-fed and protein-restricted groups of rats.
Acute heat exposure decreased the GSH content of erythrocyte significantly in rats fed an 18% protein diet while in protein-restricted group of rats, the decrease in GSH content was not statistically significant (Table 3B). Supplementation of ascorbic acid restored completely the GSH content of erythrocyte of adequately protein-fed rats following acute exposure to heat.
The membrane total thiol (-SH) content unlike the GSH content of erythrocytes increased in both adequately protein-fed and protein-restricted groups of rats following chronic exposure to heat (Table 3A). Prior supplementation of ascorbic acid potentiated the increase in membrane total thiol (-SH) content in rats, fed an 18% or a 6% protein diet.
Following acute heat exposure no marked alteration in membrane total thiol level was observed in rats fed an 18% protein diet, but in protein-restricted group of rats, it tended to be increased slightly (Table 3B). The supplementation of ascorbic acid was found to increase the reserve of membrane total thiol (-SH) content in rats fed an 18% or a 6% protein diet (Table 3B).
Iodine Number of Erythrocyte Membrane Lipids
Iodine number of membrane lipids decreased significantly in both 18% protein-fed and 6% protein-fed rats following chronic exposure to heat (Table 3A).Acute heat exposure decreased the iodine number in adequately protein-fed rats only, while in protein-restricted rats no marked change was observed (Table 3B).
Discussion
Alteration of ascorbic acid status of blood after heat exposure may be considered as an indication of heat-induced oxidative threat (Tanaka et al., 1997) [34]. The rate of entry and exit of dehydroascorbate to and from erythrocytes is more than 10 fold greater than that of ascorbate (May et al., 1995) [35]. So, higher the oxidant stress in plasma, the greater will be the conversion of plasma ascorbate and the entry of dehydroascorbate into the erythrocytes. Inside the erythrocytes, dehydroascorbate is converted to ascorbate to increase the intracellular concentration of ascorbic acid. In the present experiment, the reduction of plasma ascorbic acid level (Table 1) correlated with the above phenomenon, and thereby tended to maintain ascorbic acid concentration of erythrocytes but the decrease in ascorbic acid content (Table 1) of erythrocytes after acute heat exposure in protein-restricted rats cannot be explained with certainty.
The increased values of malondialdehyde (MDA), conjugated diene and alanine (Tables 3A and 3B), as noted in the present studies, were the outcome of heat-induced oxidative assault. Again, lipid peroxidation is a free radical-mediated oxidative deterioration of polyunsaturated fatty acid residues in membrane lipids, resulting in the formation of lipid hydroperoxide (Wayner et al., 1987) [36]. Malondial- dehydes, the peroxidative end product of unsaturated fatty acids, are water soluble; when they are free they could dissipate from erythrocyte into the plasma (Jain, 1988) [37]. Thus, MDA detected in the membrane probably is not all that was formed during the in vivo exposure of erythrocytes to heat.
Ascorbic acid has been suggested to be an effective antioxidant, either directly by reacting with aqueous radicals or indirectly by regenerating vitamin E, which is the primary scavenger of radicals within the membrane (Frei et al., 1989[38]). In spite of this action of ascorbic acid, higher values of lipid peroxidation obtained in the present study even after supplementation of ascorbic acid are not clear. However, the inability of ascorbic acid to prevent membrane lipid peroxidation is possibly due to its hydrophilic property. This property makes it unable to prevent lipid peroxidation in membrane effectively (Krous et al., 1997) [39].
The reduced glutathione (GSH) content and membrane SH-groups (Tables 4A and 4B) are important indicators of antioxidant reserve of the cell. The reduced glutathione participates in the protection of the sulphhydryl group of cysteine in protein and other cell constituents, and also in the protection of cells against oxidation by free radicals and reactive oxygen intermediates (Fujii et al., 1984; Meister, 1985) [40,41]. Heat stress induces generation of oxidizing agents, which damage the erythrocyte membrane through SH-group oxidation thus modifying the cell permeability, and inactivating the membrane bound enzymes (Bono et al., 1987) [42]. The depletion of GSH leads to lipid peroxidation and, consequently cellular damage. In the present study, GSH levels (Tables 4A and 4B) in erythrocyte were found to be decreased significantly by chronic as well as acute heat exposure. Such decrease in the GSH level in erythrocyte membrane following chronic heat exposure was further accentuated by dietary protein deficiency. On the other hand, acute heat exposure on a protein-restricted diet was found to have no significant effect on GSH level of erythrocyte membrane. This shows that dietary protein deficiency produces differential response of the membrane GSH level between chronic heat exposure and acute heat exposure. Although we have not measured the oxidized form of glutathione (GSSG), it may be suggested that during chronic exposure of higher temperature some oxidizing agents were generated and GSH was used to eliminate these agents, resulting in its diminished content in the erythrocyte membrane. The changes in erythrocyte GSH level after chronic heat exposure were restored partially and those after acute heat exposure were restored completely by ascorbic acid supplementation. This shows ascorbic acid supplementation can appreciably counteract the heat-induced changes in membrane GSH level. In spite of the diminished GSH content of the erythrocyte membrane, there was increased level of membrane SH-groups after heat exposure. Whether, this is due to increased uptake of cystine or due to other mechanisms remains to be explored by further studies. But, the increased levels of membrane GSH and SH-groups as a result of ascorbic acid supplementation (Tables 4A and 4B) is supported by the studies of May et al. (1995), who demonstrated that after supplementation of any antioxidant, the total glutathione and protein-bound glutathione generally increased, whereas non-protein glutathione significantly decreased[35].
Various reports (Ohtsuka et al., 1994) and the results of the present study emphasize that the erythrocytes have to face a great challenge against oxidative threat during in vivo exposure to heat [43]. The higher MDA, conjugated diene and alanine values in erythrocytes of heat-stressed rats were an indication of the depressed antioxidative defence mechanism. Thus, the supplementation of ascorbic acid, demonstrate that heat stress destabilizes the antioxidant defence system of erythrocyte and ascorbic acid tries to overcome it. The results further indicate that the depression of defence system and enhanced membrane lipid peroxidation were generally correlated with the degree of protein deficiency, as the extent of above changes following exposure to heat appeared comparatively greater in dietary protein in adequacy than in dietary protein adequacy.
Conclusion
In the present experiment the reduction of plasma ascorbic acid in response to chronic heat exposure is correlated with the development of heat-induced oxidative threat . But the decrease in ascorbic acid content of erythrocytes after acute heat exposure in protein-restricted rats cannot be explained with certainty. The increased values of malondialdehyde, conjugated diene and alanine as noted in the present studies were the outcome of heat-induced oxidative assault where restriction of protein in the diet aggravates the change. In the present study, GSH levels in erythrocyte were found to be decreased significantly by chronic as well as acute heat exposure. The decreased GSH level in erythrocyte membrane following chronic heat exposure, unlike acute heat exposure was further accentuated by dietary protein deficiency. This shows that dietary protein level produces differential response of the membrane GSH level between chronic heat exposure and acute heat exposure. It is further suggested that the diminished GSH level in erythrocyte membrane following exposure to heat in vivo results from increased utilization of GSH for elimination of oxidizing agents generated due to exposure to heat. The present studies also revealed that the changes in erythrocyte GSH level after chronic heat exposure were restored partially and those after acute heat exposure were restored completely by ascorbic acid supplementation. This shows ascorbic acid supplementation can appreciably counteract the heat-induced changes in membrane GSH level. The reduction of iodine number as observed in the present study favours the elimination of chance of heat-induced hyperfluidity of the membrane. This suggests that, following chronic exposure to heat, a sort of adaptive mechanism is developed in the erythrocyte membrane for maintenance of fluidity within normal level. The results further indicate that the depression of defence system and enhanced membrane lipid peroxidation were generally correlated with the degree of protein deficiency, as evident from comparatively greater changes of such parameters in protein restriction, than in protein adequacy. It is further concluded that some of detrimental effects induced by heat stress on erythrocyte membrane can be modulated by the dietary protein deficiency and can be partially or completely counteracted by the supplementation of ascorbic acid.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgement
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. We are grateful to the University Grants Commission, Government of India for funding this research work. We also acknowledge the great help and supporting assistance, received from the laboratory and library staff of theBiochemistry and Nutrition Laboratory, Department of Physiology, University of Calcutta, 92 APC Road, Kolkata-700009.
References:
1. Çimen MYB. Free radical metabolism in human erythrocytes. Clinica Chimica Acta 2008; 390 (1-2): 1-11.
[2] Chiu D, Lubin B, Shohet SB. Peroxidative reactions in red cell biology, in Pryor W (ed): Free Radicals in Biology, vol. 5. San Diego, CA, Academic, 1982; p 115.
[3] Bernabucci U, Ronchi B, Lacetera N, NardoneA.Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season.J Diary Science 2002; 85(9): 2173-2179.
[4] Ohtsuka Y, Yabunaka N, Fujisawa H, Watanabe I. Effect of thermal stress on glutathione metabolism in human erythrocytes. EurJ Appl Physiol Occup Physiol 1994; 68(1) : 87-91.
[5] Ait-Boulahsen A, Garlich JD, Edens FW. Calcium deficiency and food deprivation improve the response of chickens to acute heat stress. J Nutr 1993; 123 (1) : 98-105.
[6] Young KM, Cramp RL, Franklin CE. Each to their own: skeletal muscles of different function use different biochemical strategies during aestivation at high temperature. J Exp Biol2013;216 : 1012-1024.
[7] Altan O, Pabuçcuo?lu A, Altan A, Konyalio?lu S, Bayraktar H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers.Br Poult Sci. 2003;44(4) : 545-550.
[8] Chihuailaf RH, Conteras PA, Wittwer FG. Pathogenesis of oxidative stress: Consequences and evaluation in animal health. Vet Mex 2002; 33(3) : 265-283.
[9] Tkaczyk J, Vízek M. Oxidative stress in the lung tissue--sources of reactive oxygen species and antioxidant defence. Prague Med Rep. 2007; 108(2):105-14.
[10] Adenkola AY, Ayo JO. Effect of ascorbic acid on diurnal variations in rectal temperature of indigenous turkeys during the hot-dry season.Int J Poult Sci. 2009; 8(5):457- 461.
[11] McClung JM, Judge AR, Powers SK, Yan Z. p 38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol 2010; 298(3) : 542-549.
[12] Smuder AJ, Hudson MB, Nelson WB, Kavazis AN, Powers SK. Nuclearfactor-κBsignaling contributes to mechanical ventilation-induced diaphragm weakness. Crit Care Med2012;40 : 927-934.
[13] Whidden M A, Smuder A J, Wu M, Hudson M.B, Nelson WB, Powers SK. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J ApplPhysiol 2010;108 : 1376-1382.
[14] Dodd S L, Gagnon B J, Senf SM, Hain B A, Judge AR. Ros-mediated activation of NF-kappa B and Foxo during muscle disuse. Muscle Nerve 2010; 41: 110-113.
[15] Feng H, Juan L, Zewen L, Chia CC, Wenge Y, Li Z.Redox Mechanism of Reactive Oxygen Species in Exercise. Front Physiol 2016; 7: 486.
[16] Filomeni G, Zio DD, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death and Differentiation 2015; 22: 377-388.
[17] Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 2014; 15:81-89.
[18] Pietrocola F, Izzo V, Niso-Santano M, Vacchelli E, Galluzzi L, Maiuri MC et al. Regulation of autophagy by stress- responsive transcription factors. Semin Cancer Biol 2013 23: 310-322.
[19] Mujahid A, Akiba Y, Toyomizu M, Olive oil-supplemented diet alleviatesacute heat stress-induced mitochondrial ROS production in chicken skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2009; 297: 690-698.
[20] Cheng JZ, Sharma R, Yang Y, Singhal SS, Sharma A, Saini MK, Sing SV, Zimniak P, Awasthi S, Awasthi YC. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5.8 is an early adaptive response of cells to heat and oxidative stress. J BiolChem 2001; 276 :41213-41223.
[21] López-Revuelta A, José I, Sánchez-Gallego JI, Hernández-Hernández A, SánchezYagüe J, Marcial Llanillo M. Membrane cholesterol contents influence the protective effects of quercetin and rutin in erythrocytes damaged by oxidative stress. Chem Biol Interact 2006; 16 (1) :79-91.
[22] José I, Sánchez-Gallego, López-Revuelta A, José L, Sardina Hernández Hernández A, Sánchez-Yagüe J, Llanillo M. Membrane cholesterol contents modify the protective effects of quercetin and rutin on integrity and cellular viability in oxidized erythrocytes. Free Rad Biol Med. 2010; 48(10) : 1444-1454.
[23] Frei B, England L, Ames, BN. Ascorbate is an outstanding antioxidant in human blood plasma. ProcNatlAcadSci 1989; 86: 6377-6381.
[24]Candan F, Gultekin F, Candan F. Effect of vitamin C and zinc onfragility and lipid peroxidation in zinc- deficient haemodialysis patient. Cell Biochem Function 2012; 20 :
[25] Sunil Kumar BV, Kumar A, Kataria M. Effect of heat stress in tropical livestock and different strategies for its amelioration. J Stress Physiol Biochem 2011;7(1) :45-54.
[26]Sharma S, Zingde SM, Gokhale SM .Identification of human erythrocyte cytosolic proteins associated with plasma membrane during thermal stress. Membr Biol 2013; 246(8) : 591-607.
[27]Balogh G, Peter M, Glatz A, Gombos I, Torok Z, Horvath I, Harwood JL, Vigh L. Key role of lipids in heat stress management. FEBS Lett 2013; 587(13) : 1970-1980.
[28] West JW. Nutritional strategies for managing the heat stressed dairy cows. J Anim Sci 1999; 77(2) : 21-35.
[29] Chatterjee AK, Sadhu U, Dalal BB. Chatterjee T. Studies on certain drug metabolising enzymes in deoxypyridoxi rats. Japanese J Pharmacol 1984; 34:367- 373.
[30] Hawk PB, Oser BL. Science 1931; 74 : 369-369.
[31] Vincent T Marchesi, George E Palade. The localization of Mg-Na-K-activate adenosine triphosphatase on red cell ghost membranes. J Cell Biol 1967 35(2):385-404.
[32] Shakir FK, Audilet D, Drake AJ, Shakir KM. A rapid protein determination by modification of the Lowry procedure.Anal Biochem 1994; 216(1) : 232-233.
[33] Fisher RA, Yates F. Statistical Tables for Bio-logical, Agricultural and Medical Research, 6th ed. London: Longman Group Ltd., 1974; p 1-729.
[34] Tanaka K, Hashimota T, Tokumaru S, Iguchi H, Kajo S. Interactions between vitamin C and vitamin E are observed in tissues of inherently scorbutic rats. J Nutr 1997; 127(10):2060-2064.
[35] May JM, Qu ZC, Whitesell RR. Ascorbic acid recycling enhances the antioxidant reserve of human erythrocytes. Biochemistry 1995; 34(39):12721-12728.
[36] Wayner DM, Burton GW, Ingold KU, Barclay LR, Locke SJ. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta 1987; 924(3): 408-419.
[37] Jain SK. Evidence for membrane lipid peroxidation during the in vivo aging of human erythrocytes.Biochim Biophys Acta 1988; 937: 205-210.
[38] Frei B, England L and Ames BN. Ascorbateis an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci 1989; 86(16): 6377-6381.
[39] Krous A, Roth HP and Kirchgessner M (1997)Supplementation with vitamin C, vitamin E or beta-carotene influences osmotic fragility and oxidative damage of erythrocytes of zinc-deficient rats. J Nutr 1997; 127(7):1290-1296.
[40] Fujii S, Dale GL and Beutler E. Glutathione-dependent protection against oxidative damage of the human red cell membrane.Blood 1984; 63(5): 1096-10101.
[41] Meister A. Methods for the selective modification of glutathione metabolism and study of glutathione transport. Methods Enzymol 1985;113: 571-585.
[42] Bono A, Caimi G, Catania A, Sarsno A, PandolfoL Red cell peroxide metabolism in diabetes mellitus. Horm Metab Res 1987; 19(6):264-266.
[43]Ohtsuka Y, Yabunaka N, Fujisawa H, Watanabe I. Effect of thermal stress on glutathione metabolism in human erythrocytes. Eur J Appl Physio Occup Physiol 1994;68(1) : 87-91.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License