IJCRR - 2(1), January, 2010
Pages: 17-27
Print Article
Download XML Download PDF
DERIVATIVE SPECTROPHOTOMETRIC ESTIMATION OF NEVIRAPINE IN BULK DRUG AND
PHARMACEUTICAL DOSAGE FORMS
Author: Jawade S. K., Khanage S.G., Mohite P.B., Deshmukh V.K.
Category: Healthcare
Abstract:A simple, accurate and precise spectrophotometric method has been developed and validated for the
estimation of nevirapine from bulk drug and tablet formulations. Nevirapine shows a sharp peak at
241.0 nm in first order derivative spectrum with n =1. The drug follow Beer-Lambert?s law in the
concentration range of 4-24 \?g/ml in this method. Result of the analysis was validated statistically and found
accurate. The method was successfully applied for determination of drug in tablets, wherein no
interference from tablet excipients was observed, indicating the specificity of the developed method. Thus the
proposed method can be used successfully for routine analysis of nevirapine from capsule and tablet
formulations.
Keywords: Nevirapine, Ultraviolate Spectroscopy, Derivative spectroscopy, validation, precise, accurate .
Full Text:
INTRODUCTION
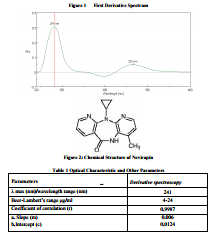
Nevirapine (Fig.1), 11-cyclopropyl-4- methyl-5, 11-dihydro-6H-dipyrido [3, 2-b: 2?, 3?- e][1,4] diazepin-6-one is a reverse transcriptase (RT) inhibitor of human immunodeficiency virus type 1 (HIV-1)1,2. Nevirapine inhibits replication of HIV-1 by interfering with viral RNAdirected DNA polymerase (reverse transcriptase). It binds directly to herodimeric HIV-1 reverse transcriptase and exerts a virustatic effect by acting as a specific, noncompetitive HIV-1 reverse transcriptase inhibitor; it appears to inhibit viral RNA- and DNAdependent DNA polymerase activities by disrupting the catalytic site of the enzyme3 . Literature survey reveals that there are analytical methods available for determination of nevirapine from biological matrices 4-14, bulk drug and dosage forms 15-17, and analytical methods for determination of nevirapine with combination of other antiviral drugs 18-52 Literature survey further revealed that there were very few reported RP-HPLC and spectrophotometric estimation methods for the analysis of nevirapine. Thus, an appropriate analytical procedure for the quantitative determination of nevirapine from bulk drugs is of considerable importance. Keeping this objective in mind an attempt has been made to develop and validate UV estimation method for the analysis of nevirapine which would be highly sensitive, having good resolution and reproducible. Various validation aspects of the analysis, accuracy, precision, recovery and the limits of detection and quantification etc., have been measured.
Material and Methods The working standard of nevirapine was procured from Cipla Ltd., India. analytical grade Hydrochloric acid. 0.1N HCl prepared in distilled water used as solvent. JASCO V-630 UV/VIS spectrophotometer was used with 1cm matched quartz cells. Tablets of 200 mg strength were procured from local pharmacy (Nevimune-200). Accurately about 100mg of the pure drug was weighed and dissolved in sufficient quantity of 0.1N HCl and volume made up to100ml with 0.1N HCl to give standard stock solution (1
RESULTS AND DISCUSSION
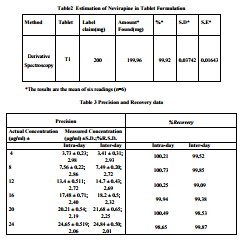
The solvent chosen for UV must take into account the chemical nature and polarity of the drug molecule. Nevirapine is practically insoluble in water, soluble in 0.1 N hydroclodic acid, dichloromethane, dimethylsulphoxide and dimethylformamide, slightly soluble in methanol. Calibrator solutions were prepared in 0.1N HCl. The method developed was validated for limit of detection (LOD) and limit of quantitation (LOQ) in order to determine and ensure sensitivity of the developed method. The method was found to be linear over the range 4-24 μg/ml per spot with coefficient of mg/ml). Aliquots of standard stock solution were pipette out and suitably diluted with 0.1N HCl to get final concentration of 4-24μg/ml of standard solution. The solution were scanned in the spectrum mode from 400 nm to 200 nm wavelength range and the first order derivative spectra were obtained at n =1 (Method A) a sharp peak was obtained at 241nm (Figure-1). The absorbance difference at n=1 (dA/dλ) was calculated by the inbuilt software of the instrument which is directly proportional to the concentration of the standard solution .A calibration curve was plotted taking the absorbance difference (dA/dλ) against the concentration of the standard solutions. The method was applied for the sample solution of known concentration and was found be satisfactory for analysis of tablet formulation.
correlation 0.9987. (Table 1) Intra-day and inter-day precision studies showed a % RSD was less than 5.00%, indicating the method was precise. The accuracy values obtained, in the range 98.53 – 100.73 % for drug are indicative of excellent accuracy and recovery. This indicates the method is specific. Stability studies were carried out for standard. It was found to be stable in sample solution, prior to development and after development. The developed method was then validated and successfully applied for quantitation of nevirapine from the formulation. To ensure accuracy of the method, recovery studies were performed by standard addition method at 80%, 100% and 120% level,
to the pre-analyzed samples and the subsequent solutions were re-analyzed. At each level, three determinations were performed and the results obtained are shown in Table 3. The results of recovery studies were within the specified limits of ICH guidelines54. Lower values of %RSD reflect the accuracy of the method. Precision, expressed in terms of %RSD was determined in terms of intra-day and interday precisions, analyzing the drug at six different concentrations, determining each concentration thrice. The sample solutions were analyzed using the method for 3 consecutive days, repeating the process twice a day at different period. The results obtained are summarized in Table 3 and reflect high degree of precision. Two different analysts performed assay on marketed tablets of the drug, in similar operational and environmental conditions, using the developed method to determine its ruggedness. A typical absorbance spectrum of the drug is shown in Fig.1.
CONCLUSION
The developed and validated UV estimation method reported here is rapid, simple, accurate, sensitive and specific. The method was also successfully used for quantitative estimation and analysis of Nevirapine from formulation. Thus the reported method is of considerable importance and has great industrial applicability for quality control and analysis of Nevirapine from bulk drug and formulations.
ACKNOWLEDGEMENT
Authors thank Cipla Ltd., India for supplying the authenticated standard of Nevirapine and Mr. Ajay Pise for his moral support. Also thanks to M.E.S. College of Pharmacy for providing the requirements.
References:
1 Indian Pharmacopoeia (2007), Vol 3, Government of India Ministry of Health and Family Welfare, published by The Indian Pharmacopoeia Commission, Ghaziabad, pp 1433- 1434.
2 USP-NF (2009), Vol III, USP-NF The Official Compendia of Standards, published by The United States Pharmacopoeia Convention, City Press, Baltimore, US, pp 3072-3073.
3 Mirochnick M, Clarke DF, Dorenbaum A. (2000) Nevirapine: Pharmacokinetic considerations in childrens and pregnant women. Clin. Pharmacokinet., 39, 281–293.
. 4. Silverthorn C.F., Parsons T.L., (2006) Biomed. Chromatogr., 20, 23- 27.
5. Bennetto C.J., King J.R., Turner M.L., Stringer J.S.A., Acosta E.P., (2004) A validated new method for nevirapine quantitation in human plasma via high-performance liquid chromatography. Clin.Chem., 50, 209- 211.
6. Langmann P., Schirmer D., Vath T., Desch S., Zilly M., Klinker H., (2002) Rapid determination of nevirapine in human plasma by gas chromatography. J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci. , 767, 69-74.
7. Lopez R. M., Pou L., Gomez M. R., Ruiz I., Monterde J., (2001) Simple and rapid determination of
nevirapine in human serum by reversed-phase high-performance liquid chromatography. J. Chromatogr. B. Biomed. Sci. and Appl., 751, 371-376.
8. Heeswijk R.P.G., Hoetelmans R.M.W., Meenhorst P.L., Mulder J.W., Beijnen J.H., (1998), Rapid determination of nevirapine in human plasma by ion-pair reversedphase highperformance liquid chromatography with ultraviolet detection. .J. Chromatogr. B. Biomed. Sci. and Appl., 713, 395-399.
9. Pav J.W., Rowland L.S., Korpalski D.J., (1999) HPLC-UV method for quantitation of nevirapine in biological matrices following solid-phase extraction. J. Pharm. Biomed. Anal., 20, 91-98.
10. Hollanders R.M.F., Ewijk-Beneken Kolmer E.W.J., Burger D.M., Wuis E.W., Koopmans P.P., Hekster Y.A.,(2000) Determination of nevirapine, an HIV-1 nonnucleoside reverse transcriptase inhibitor, in human plasma by reversed-phase highperformance liquid chromatography. J. Chromatogr. B. Biomed. Sci. and Appl., 744, 65-71.
11. Dubuisson J.G., King J.R., Stringer J.S.A., Turner M.L., Bennetto C., Acosta E.P., (2004) Detection of Nevirapine in plasma using Thin-Layer Chromatography. JAIDS Journal of Acquired Immune Deficiency Syndromes, 35, 155-157.
12. Chi J., Jayewardene A.L., Stone J.A., Aweeka F.T., (2003) An LC-MSMS method for the determination of nevirapine, a non-nucleoside reverse transcriptase inhibitor, in human plasma. J. Pharm. Biomed. Anal., 31, 953-959.
13. Laurito T.L., Santagada V., Caliendo G., Oliveira C.H., BarrientosAstigarraga R.E., De Nucci G., (2002) Nevirapine quantification in human plasma by high-performance liquid chromatography coupled to electrospray tandem mass spectrometry. Application to bioequivalence study. Journal-ofMass-Spectrometry, 37, 434-441.
14. Pattarawarapan M., Nangola S., Cressey T.R., Tayapiwatana C., (2007) Development of a one-step immune chromatographic strip test for the rapid detection of nevirapine (NVP), a commonly used antiretroviral drug for the treatment of HIV/AIDS. Talanta, 71, 462-470.
15. Kaul N., Agrawal H., Paradkar A.R., Mahadik K.R. (2005) J. Biochem. Biophys. Methods, 64, 121– 141.
16. Kaul N., Agrawal H., Paradkar A. R., Mahadik K. R., (2004) HPTLC method for determination of nevirapine in pharmaceutical dosage form. Talanta, 62, 843-852.
17. Li Q.C., Tougas T., Cohen K., Lee R., Meagan P., Corson M., Muchnick T., (2000) Validation of a HighPerformance Liquid Chromatography Method for the Assay of and Determination of Related Organic Impurities in Nevirapine Drug Substance. J. Chromatogr. Sci., 38, 246-254.
18. Aymard G., Legrand M., Trichereau N., Diquet B., (2000) Determination of twelve antiretroviral agents in human plasma sample using reversed-phase highperformance liquid chromatography. J. Chromatogr. B. Biomed. Sci. and Appl., 744, 227-240.
19. Dailly E., Raffi F., Jolliet P., (2004) Determination of atazanavir and other antiretroviral drugs (indinavir, amprenavir, nelfinavir and its active metabolite M8, saquinavir, ritonavir, lopinavir, nevirapine and efavirenz) plasma levels by high performance liquid chromatography with UV detection. J. Chromatogr. B., 813, 353–358.
20. Poirier J.M., Robidou P., Jaillon P., (2005) Simple and Simultaneous Determination of the HIV-Protease Inhibitors Amprenavir, Atazanavir, Indinavir, Lopinavir, Nelfinavir, Ritonavir and Saquinavir Plus M8 Nelfinavir Metabolite and the Nonnucleoside Reverse Transcriptase Inhibitors Efavirenz and Nevirapine in Human Plasma by Reversed-Phase Liquid Chromatography. Ther. Drug. Monit., 27, 186–192.
21. Rezk N.L., Tidwell R.R., Kashuba A.D.M., (2002) Simple and rapid quantification of the non-nucleoside reverse transcriptase inhibitors nevirapine, delavirdine, and efavirenz in human blood plasma using highperformance liquid chromatography with ultraviolet absorbance detection. J. Chromatogr. B., 774, 79–88.
22. Rezk N.L., Tidwell R.R., Kashuba A.D.M, (2004) High-performance liquid chromatography assay for the quantification of HIV protease inhibitors and nonnucleoside reverse transcriptase inhibitors in human plasma. J. Chromatogr. B., 805, 241– 247.
23. Notari S., Bocedi A., Ippolito G., Narciso P., Pucillo L.P., Tossini G., Donnorso R.P., Gasparrini F., Ascenzi P.(2006) Simultaneous determination of 16 anti-HIV drugs in human plasma by high-performance liquid chromatography. J. Chromatogr. B., 831, 258–266.
24. Simon V.A., Thiam M.D., Lipford L.C., (2001) Determination of serum levels of thirteen human immunodeficiency virus-suppressing drugs by high-performance liquid chromatography. J. Chromatogr. A., 913, 447–453.
25. Titier K., Lagrange F., Pehourcq F., Edno M.L., Moore N., Molimard M., (2002) High- Performance Liquid Chromatographic Method for the Simultaneous Determination of the Six HIV-Protease Inhibitors and Two NonNucleoside Reverse Transcriptase Inhibitors in Human Plasma. Ther. Drug. Monit., 24, 417-424.
26. Rezk N. L., Crutchley R.D., Yeh R. F., Kashuba A. D. M., (2006) Full Validation of an Analytical Method for the HIV-Protease Inhibitor Atazanavir in Combination with Other Antiretroviral Agents and its Applicability to Therapeutic Drug Monitoring. Ther. Drug. Monit., 28, 517-525.
27. Tribut O., Verdier M.C., Arvieux C., Allain H., Michelet C., Bentue F. D., (2005) Simultaneous Quantitative Assay of Atazanavir and 6 Other HIV Protease Inhibitors by Isocratic Reversed-Phase Liquid Chromatography in Human Plasma. Ther. Drug. Monit., 27, 265-269.
28. Tribut O., Arvieux C., Michelet C., Chapplain J.M., Allain H., Bentue F.D., (2002) Simultaneous Quantitative Assay of Six HIV Protease Inhibitors, One Metabolite, And Two Non-Nucleoside Reverse Transcriptase Inhibitors in Human Plasma by Isocratic Reversed-Phase Liquid Chromatography. Ther. Drug. Monit., 24, 554–562.
29. Kappelhoff B.S., Rosing H., Huitema A.D.R, Beijnen J.H. (2003) Simple and rapid method for the simultaneous determination of the nonnucleoside reverse transcriptase inhibitors efavirenz and nevirapine in human plasma using liquid chromatography. J. Chromatogr. B., 792, 353–362
. 30. Colombo S., Beguin A., Telenti A., Biollaz J., Buclin T., Rochat B., Decosterd L.A., (2005) Intracellular measurements of anti-HIV drugs indinavir, amprenavir, saquinavir, ritonavir, nelfinavir, lopinavir, atazanavir, efavirenz and nevirapine in peripheral blood mononuclear cells by liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B., 819, 259–276.
31. Rentsch K.M., (2003) Sensitive and specific determination of eight antiretroviral agents in plasma by highperformance liquid chromatography– mass spectrometry. J. Chromatogr. B., 788, 339–350.
32. Villani P., Feroggio M., Gianelli L., Bartoli A., Montagna M., Maserati R., Regazzi M.B., (2001) Antiretrovirals: Simultaneous Determination of Five Protease Inhibitors and Three Nonnucleoside Transcriptase Inhibitors in Human Plasma by a Rapid High- erformance Liquid Chromatography-Mass Spectrometry Assay. Ther. Drug. Monit., 23, 380–388.
33. Lemmer P., Schneider S., Schuman M., Omes C., Arendt V., Tayari J.C., Fundira L., Wennig R., (2005) Determination of Nevirapine and Efavirenz in Plasma Using GC/MS in Selected Ion Monitoring Mode. Ther. Drug. Monit., 27, 521-525.
34. Therese K., Heike B., Regina R., Michal S., Klaus R., Volkhard K., (2005) Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry, 19, 2995-3001.
35. Benet. L.Z., Christians U., EggeJacobsen W., Unger M., Niemann C.U., Baluom M., Hirai S., (2004) Automated, Fast, and Sensitive Quantification of Drugs in Human Plasma by LC/LC-MS: Quantification of 6 Protease Inhibitors and 3 Nonnucleoside Transcriptase Inhibitors. Ther. Drug. Monit., 26, 546-562 36. Pereira E.A., Micke G.A., Tavares M.F.M, (2005) Determination of antiretroviral agents in human serum by capillary electrophoresis. J. Chromatogr. A., 1091, 169-176.
37. Tuan N.D., Wolfgang G., Karin S., Heribert S., Barbara F., Manfred P.D., Andreas Z., (2003) Simultaneous separation of fifteen approved protease and reverse transcriptase inhibitors for human immunodeficiency virus therapy by capillary electrophoresis. Electrophoresis, 24, 662-670.
38. Fan B., Stewart J.T., (2001) Determination of zidovudine/zalcitabine/nevirapine in human plasma by ion-pair HPLC. J. Liq. Chrom. Relat. Tech., 2001, 24, 3017-3026.
39. Narang V.S., Lulla A., Malhotra G., Purandare S., (2005) A CombinedFormulation Tablet of Lamivudine/Nevirapine/Stavudine: Bioequivalence Compared With Concurren Administration of Lamivudine, Nevirapine, and Stavudine in Healthy Indian Subjects. J. Clinical. Pharmacology., 45, 265- 274.
40. Dailly E., Thomas L., Kergueris M. F., Jolliet P., Bourin M., (2001) Highperformance liquid chromatographic assay to determine the plasma levels of HIV-protease inhibitors (amprenavir, indinavir, nelfinavir, ritonavir and saquinavir) and the non-nucleoside reverse transcriptase inhibitor (nevirapine) after liquid–liquid extraction. J. Chromatogr. B. Biomed. Sci. and Appl. 758, 129-135.
41. Droste J.A.H., Verweij-van W.C.P.W.G.M., Burger D.M., (2003) Simultaneous Determination of the HIV Drugs Indinavir, Amprenavir, Saquinavir, Ritonavir, Lopinavir, Nelfinavir, the Nelfinavir Hydroxymetabolite M8, and Nevirapine in Human Plasma by Reversed-Phase High-Performance Liquid Chromatography. Ther. Drug. Monit., 25, 393-399.
42. Fan B., Stewart J.T., (2002) Determination of zidovudine/lamivudine/nevirapine in human plasma using ion-pair HPLC. J. Pharm. Biomed. Anal., 28, 903-908.
43. Rezk N.L., Tidwell R.R., Kashuba A.D.M., (2003) Simultaneous determination of six HIV nucleoside analogue reverse transcriptase inhibitors and nevirapine by liquid chromatography with ultraviolet absorbance detection. J. Chromatogr. B., 791, 137-147.
44. Marzolini C., Beguin A., Telenti A., Schreyer A., Buclin T., Biollaz J., Decosterd L. A., (2002) Determination of lopinavir and nevirapine by highperformance liquid chromatography after solid-phase extraction: application for the assessment of their transplacental passage at delivery. J. Chromatogr. B., 774, 127-140.
45. Marchei E., Valvo L., Pacifici R., Pellegrini M., Tossini G., Zuccaro P., (2002) Simultaneous determination of zidovudine and nevirapine in human plasma by RP-LC. J. Pharm. Biomed. Anal., 29, 1081-1088.
46. Ramachandran G., Hemanthkumar A.K., Kumaraswami V., Swaminathan S. (2006) A simple and rapid liquid chromatography method for simultaneous determination of zidovudine and nevirapine in plasma. J. Chromatogr. B., 843, 339-344.
47. Gutleben W., Scherer K., Tuan N.D., Stoiber H., Dierich M.P., Zemann A., (2002) Simultaneous separation of 11 protease and reverse transcriptase inhibitors for human immunodeficiency virus therapy by co-electroosmotic capillary zone electrophoresis. J. Chromatogr. A., 982, 153-161
48. Fan B., Stewart J.T., (2002) Determinations of zidovudine/didanosine/nevirapine and zidovudine/didanosine/ritonavir in human serum by micellar electrokinetic chromatography. J. Pharm. Biomed. Anal., 30, 955-960.
49. Anbazhagan S., Indumathy N., Shanmugapandiyan P., Sridhar S.K., (2005) Simultaneous quantification of stavudine, lamivudine and nevirapine by UV spectroscopy, reverse phase HPLC and HPTLC in tablets. J. Pharm. Biomed. Anal., 39, 801-804.
50. Sarkar M., Khandavilli S., Panchagnula R., (2006) Development and validation of RPHPLC and ultraviolet spectrophotometric methods of analysis for the quantitative estimation of antiretroviral drugs in pharmaceutical dosage forms. J. Chromatogr. B., 830, 349-354
. 51.Hamrapurkar P.D.*, Phale M. D., Shah N., (2009)Quantitative Estimation Of Nevirapine By High Performance Thin Layer Chromatography. J. Pharm. Research and H. Care., vol. 1, no.2, 197-216.
52. Venugopal K. ; Srinivasa Rao Y.; Nagogik. E. V.; Haritha G. ; Seshgirarao J. V. L. N(2005) A reversed phase high performance liquid chromatographic method for estimation of nevirapine in tablets. Indian J. Pharm. Sci., vol 63, no.1,130- 132.
53. Palanippan M., Sarkar D., Chaudhary T., Gauthaman K.(2008) A Simple and Rapid RP-HPLC Method for the Estimation of Nevirapine in Bulk and Pharmaceutical Dosage Forms. E-J Pharm., vol. 5, 1081-1086.
54. ICH [Validation of Analytical Procedures: Methodology (Q2B)], International conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, 1997 and August 2002.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License