IJCRR - 8(13), July, 2016
Pages: 06-11
Date of Publication: 12-Jul-2016
Print Article
Download XML Download PDF
EFFECT OF DIFFERENT PROCESSING METHODS ON POLYPHENOLIC CONTENT AND ANTIOXIDANT ACTIVITY OF BROAD BEANS (VICIA FABA)
Author: Pinki Saini, Priyanka Singh, Shreyasi Dubey, and Ayushi Srivastava
Category: Healthcare
Abstract:Objective: The effects of processing on total phenolic components and antioxidant activity in commonly consumed broad bean was investigated. Methods: The raw and processed samples were extracted with 70% methanol and analysed for antioxidant components and antioxidant activity.
Results: Processing of legumes caused decrease in total phenolic content when compared to the raw samples. However, the dry heating caused remarkable increase in tannin contents (6.98\?0.53 g TAE/100 g extract). The flavanoid and \? carotene content was significantly reduced on processing of samples. Raw sample of D. lablab was found to possess the highest DPPH (73.5\?2.5%), Reducing power (4.9\?0.68 mg ascorbic acid/gm) and Iron chelating capacity than other samples. Conclusion: Maximum retention of antioxidant activity was observed in dry heated samples. Higher correlation was found between phenolic content and chelating capacity (r2=0.945) but a poor correlation with DPPH. Moreover, the content of tannins gave good correlation (r2=0.745\?0.913) with Iron chelating and DPPH assays.
Keywords: Processing, Antioxidant, Broad beans, Total phenolics, Correlation
Full Text:
INTRODUCTION
Legumes belong to the family Leguminosae, one of the most important families in Dicotyledons, including around 700 genera and 20,000 species. Legumes are the second most important source of food and fodder, green manures and forages. In comparison of cereal grains, legumes are good source of proteins, dietary fibers, low glycemic indexes, low levels of fat (2-5%) and high amounts of carbohydrates (55- 60%) (Xu et al., 2007). Recently demand for plant based proteins has increased and hence there are more studies on functional proteins from legumes such as chickpea, lentil, cowpea, lupins, pea and broad beans as alternative to soybean. The epidemiological evidence indicates that the consumption of dietary antioxidant such as legume seed proteins provided protective effects for several chronic diseases like cardiovascular diseases, cancer, obesity diabetes and hypercholesterolemia. Broad beans also known as the field bean or fava bean is a species of bean native to North Africa and extensively cultivated in south and south west Asia. In India, it is an important legume used as a pulse and vegetable for human consumption and forage. Broad beans (Vicia faba) are a potential source of protein, dried seeds of bean contain 20–28% crude protein and the amino acids are moderately well balanced with especially high lysine content. They are rich in Ldopa, a substance used medically in the treatment of Parkinson’s disease. L-dopa is also a natriuretic agent, which might help in controlling hypertension. An antifungal protein Dolichin, has also been purified from the seeds of the field bean. Dolichin inhibited Human Immuno-deficiency Virus (HIV) reverse transcriptase, D and E-glucosidases which are glycohydrolases implicated in HIV infection. It had very low ribonuclease and cell-free translation-inhibitory activities (Ye et al., 2000). Further, the dietary protein concentrates of broad beans showed potential hypocholesterolemic effect (Chau et al.,. 1998).
The antioxidant activities and phenolic compounds in fresh legumes have been reported earlier in several communications (Amarowicz et al., 2003; Xu et al., 2007). However the legumes require sufficient processing before consumption. The effects of processing methods on phenolics and antioxidant activities have not been systematically studied. In addition, very little information is available in the literature regarding the changes in antioxidant activity of the processed beans. The aim of the present study is to investigate the effects of processing methods (boiling, drying and pressure cooking) on the phenolic contents and antioxidant activities of broad beans.
MATERIAL AND METHODS
Sample collection and Processing Broad beans were procured from local market of Allahabad city. They were washed, dried and stored under refrigeration. The fresh Broad beans (100g) were boiled using beans: water ratio of 1:5 (w/v) until they became tender. Pressure cooking of samples (100g) was done in a pressure cooker for 20 min with water ratio of 1:3 (w/v). Water was decanted, boiled and pressure cooked samples were dried at 50o C until constant weight reached. Another 100g of sample was dried at 160o C for 15 min in a microwave oven. The fresh, boiled, pressure cooked and dried bean samples were finely powdered using a Willy Mill of 60 mesh size. All the powdered samples were stored separately in a screw capped bottles at a room temperature until further analysis Proximate analysis: All the samples were analysed for proximate composition using AOAC (2005) methods. All the chemicals used were of analytical grade obtained from Merck or Sigma. Preparation of solvent extract: Raw and processed bean powder (100 g) was extracted with 500 ml of 70% methanol (w/v) using a shaker, the sample was shaken occasionally for 24 h. The extracts were centrifuged at 5,000 rpm for 20 min and the supernatants obtained were concentrated with a rotary vacuum evaporator (RV-10, IKA) at 45º C. The resultant extracts were stored in amber vials at 4°C until assayed. The extract recovery percentage of raw, boiled, dry heated and pressure cooked samples of Broad beans were found to be 1.66%, 1.25%, 1.50% and 1.12%, respectively. Estimation of Total Phenolic Content (TPC): Total phenolic content was determined by adopting Folin-Ciocalteu method (Velioglu et al., 1998; Ying et al., 2013). Basically, 0.2 ml of extracts was added with 1.5 ml of Folin-Ciocalteu reagent and mixture was allowed to stand at room temperature for 5 minutes. Then 1.5 ml of sodium carbonate solution (6%) was added into the mixture. Absorbance was measured using spectrophotometer at 725 nm after incubating the sample to stand for 1½ hours at room temperature. Results were expressed as gallic acid equivalent in mg/100 g dry weight (DW). Estimation of Total Tannin Content (TC): Tannin content was determined by the method of Ranganna, 2005. Powdered sample (0.5 g) was boiled with water (75ml) for 30 minutes and centrifuged at 2000 rpm for 20 minutes and the supernatant was collected. Folin Denis reagent and sodium carbonate was added to the sample extract, solution was diluted to 100ml with water and absorbance is taken at 700 nm after 30 minutes. Estimation of Total Flavonoid Content: A colorimetric assay (Kim et al., 2003) with some modification was used to quantify total flavonoid content. Briefly, 25 microliter of diluted sample was added to 125 microliter of double distilled H2 O. Subsequently, 7.5 microliter of 5% NaNO2 was added to the mixture and was allowed to stand for 5 minute thereafter 15 microliter of 10% AlCl3 was added. The mixture was incubated at ambient temperature (25o C) for an additional 5 minute. Following that and 50 microliter of 1 M NaOH was then added to the mixture. The mixture was immediately diluted by addition of 27.5 microliter of ddH2 O and the absorbance of the mixture was measured at 510 nm against a blank prepared with ddH2 O using microplate reader (synergy HT, BioTek instrument, USA). Estimation of Beta carotene: Beta carotene was analyzed by column separation method (Rangana 2005). The absorbance was measured using spectrometer at 452nm. Petroleum ether and acetone mixture was used as blank. DPPH free radical scavenging assay: The free radical scavenging activity of the field bean extracts was measured by measuring the decrease in absorbance of ethanolic DPPH solution at 517 nm in the presence of the extract (Krings and Berger, 2001; Koolen et al., 2013). The initial concentration of DPPH was 0.1 mM and the reading was taken after allowing the solution to stand for 30 min. In cases where the absorbance decreased too much before the 30 minutes period the sample was appropriately diluted. The antioxidant activity was expressed as:- Estimation of Reducing power: The reducing power of the extracts was determined by using potassium ferricyanideferric chloride method (Oyaizu, 1986). Different dilutions of extracts amounting to 1 ml were added to 2.5 ml 0.2 M phosphate buffer (pH=6.6) and 2.5 ml potassium ferricyanide (1%). The mixtures were incubated at 50°C for 20 minutes, after which 2.5 ml trichloroacetic acid (10%) was added. 2.5 ml of the mixture was taken and mixed with 2.5 ml water and 0.5 ml 1% ferric chloride. The absorbance at 700 nm was Chelating Capacity on Fe2+:- Fe2+ chelating capacity was measured by 2, 2′-bipyridyl competition assay (Yamaguchi et al., 2000). The reaction mixture contained 0.25 ml of 1 mM FeSO4 solution, 0.25 ml of sample extract, 1 ml of 0.2 M Tris–HCl buffer (pH 7.4), 1 ml of 2,2′- bipyridyl solution (0.1% in 0.2 M HCl), 0.4 ml of 10% hydroxylamine– HCl, and 2.5 ml of ethanol. The final volume was made up to 5 ml with distilled water. The absorbance at 522 nm was determined and used to evaluate Fe2+ chelating activity using ethyelendiamine tetra acetate (EDTA) as a standard. The results were expressed as mg EDTA equivalent/ g of seed extracts.
RESULTS
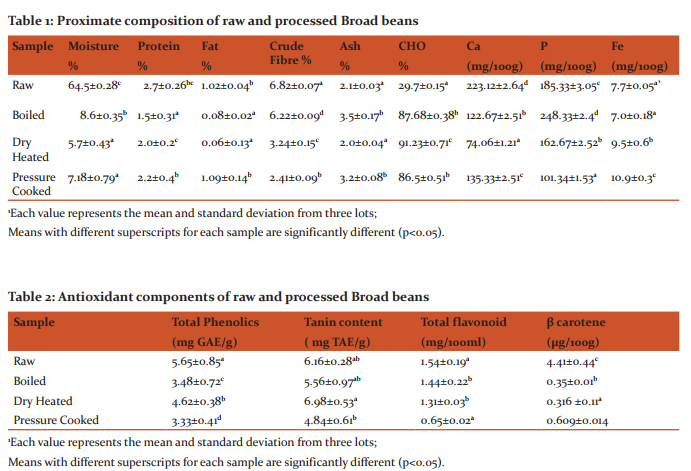
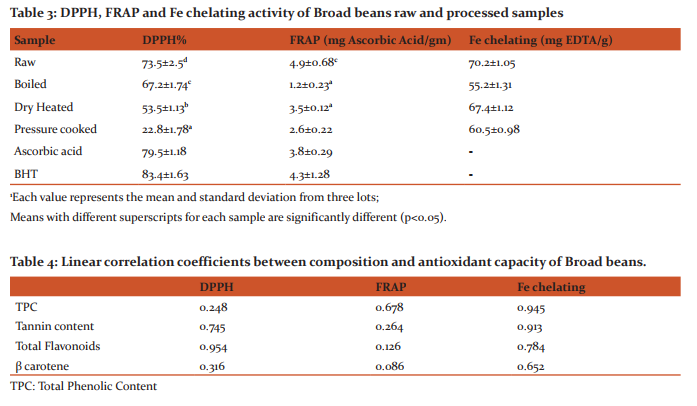
Nutritional composition of broad beans: Raw broad beans had crude protein (2.69%) and crude fat (1.02%), ash (2.1%) and crude fibre (1.82%). Raw broad beans had a high protein, crude fibre and calcium content as compared to processed field bean. The phosphorous was highest in boiled broad beans whereas iron content was highest in pressure cooked bean samples. Antioxidant components: Plant phenolics are free radical scavangers and act as antioxidants. The content of polyphenols in broad beans is depicted in Table 2. Total phenolics ranged from 3.33 to 5.65 mg GAE/g extract. The raw broad beans had highest phenolic content followed by dry heated and boiled samples. Tannin content varied from 5.13 to 6.16 mg TAE/g. Raw field bean samples had highest tannin content followed by dry heated and boiled samples. Flavonoid content was in the range of 0.65 to 1.54 mg/100ml (Table 2). Highest flavonoid content was found in raw broad beans which reduced on processing. Maximum reduction was observed in pressure cooked broad beans. The β carotene content varied from 0.31 to 4.41 µg/100g. The highest β carotene content was found in raw field bean samples followed by pressure cooked samples (0.6 µg/100g). Linear correlation coefficient between composition and antioxidant capacity of broad beans has been discussed in Table 4. High correlation coefficient was found between TPC and Iron chelating capacity (r2 =0.945) and FRAP (r2 =0.678), but a poor correlation with DPPH (r2 =0.248). Similarly tannin content showed high correlation coefficient with iron chelating capacity (r2 =0.913) and DPPH (r2 =0.745). A high correlation coefficient was observed between Total Flavanoids and DPPH (r2 =0.945) and Iron chelating capacity (r2 =0.784). β carotene showed a poor correlation with antioxidant assays (r2 =0.086-0.652). Antioxidant activity of broad beans: DPPH was used to determine the free radical scavenging activity of the methanol extracts of raw and processed field bean samples (Table 3). The raw broad beans showed highest DPPH content (73.5%) followed by boiled and dry heated samples. The pressure cooked broad beans had lowest DPPH content (22.8%), Ascorbic acid and BHT were positive controls and exhibited DPPH content as 79.5% and 83.4% respectively. Ferric ion reducing capacity: The reducing properties are related with the presence of reductones, which exert antioxidant action by breaking the free radical chain by donating a hydrogen atom (Shimada et al., 1992). Ferric ion reducing capacity of samples and standards are found to be in following order (Table 3): ascorbic acid (5.1±0.29 mg AA/ gm) > raw (4.9±0.68 mg AA/gm) >BHT (4.3± 0.54 mg AA/ gm) > pressure cooked (3.5±0.34 mg AA/gm) > dry heated (2.6±0.12 mg AA/gm) > boiled (1.2±0.23 mg AA/gm). The dry heated samples had higher reducing power than pressure cooked and boiled samples. Fe chelating: In this study, the chelating ability of the raw and processed seed sample extracts of D. lablab towards ferrous ions were examined (Table 3). All the samples examined showed Fe2+ ion chelating effect and the activity was expressed as mg EDTA equivalent. The raw samples showed a chelating capacity of 70.2±1.05 mg EDTA/g of extract followed by dry heating (67.4±1.12 mg EDTA/g). The boiled broad beans showed minimum Fe capacity (55.2±1.3 EDTA/g).
DISCUSSION
Nutritional composition: The proximate analysis of beans is given Table 1. Raw beans showed a higher nutritional value as compared to processed beans. The decrease in the ash content of processed vegetables could be as a result of processing during which some of the inorganic salt in the vegetables might have leached off (Yaciuk and Sofose 1981). The protein content also showed reduction may be due to the fact that during boiling cellular protein are denatured and the chlorophyll which is bound to protein may be released, such free chlorophyll are highly unstable and are readily converted to pheophytin which is olive green to brown in colour (Komolafe and Obayanju, 2003). Antioxidant components: The polyphenolic and tannin content of broad beans is depicted in Table 2. The results are in accordance with Pascharicha et al. (2014) who also reported total phenolic content of 22.415 GAE equivalents (μg GAE/mg sample) in faba seeds. Siddhuraju (2007) reported that processed samples had lower concentration of phenolic fractions possibly due to the poor extractability by the formation of insoluble tannin- protein and tannin-carbohydrate complexes. The Reduction of phenolic content in broad beans may be due to lixiviation (Siddhuraju and Becker 2003) and the phenols may also bound to other compounds and form insoluble complexes (Fernandez et al., 2003). Similar decrease in phenolics content of broad beans has also been reported by Maheshu et al. (2013). The results are also in accordance with Barroga et al. (1985) who found that boiling and cooking reduced the amount of phenolics in legumes by 75%. However this might be caused in part by diffusion of phenolics from the seed coat to cooking water (Rocha-Guzman et al. 2007). The total flavonoid content (Table 2) of the raw and processed samples was estimated by the aluminium chloride method. It has been recognized that flavonoids show antioxidant activity. The total flavonoid content in the dried faba seeds was estimated to be 7.814 in μg of Catechin equivalents (CE) / mg (Milo, 2004). The β-carotene content of beans was found to decrease on processing. The high sensitivity of β-carotene to light and heat is well recognized and its loss is therefore expected during heat-processing. Some workers have reported losses of β-carotene from vegetables, including spinach, amaranth and fenugreek, during cooking procedures, such as boiling, stewing, frying, blanching and pressure cooking (Yadav and Sehgal, 1995 and Yadav and Sehgal, 1997). The linear correlation between composition and antioxidant capacity of broad beans (Table 4) show that antioxidant activity is not alone dependent on total phenolics. Also the synergistic equation between antioxidants in mixture makes them dependent on concentration as well as on structure and interaction among them (Djeridane et al., 2006). The antiradical and antioxidant activities of beans depend on the amount and composition of the antioxidants they contain. The research conducted by Oomah et al. (2005) with Canadian bean cultivars revealed differences between the cultivars in antioxidant and antiradical activities. Antioxidant activity of Broad beans DPPH content was highest in raw samples followed by boiled and dry heated samples (Table 3). Saini and Singh (2015) have reported that ethanolic extracts of raw spices and herbs show higher DPPH content as compared to other extracts. The antiradical scavenging activity of untreated and treated seed extracts are related to the nature of phenolics, thus contributing to their electron transfer/hydrogen donating ability (Brand-Williams et al. 1995). According to Tsai and She (2006) a change in phenolic compounds after heating might be contributed to a decrease in DPPH-scavenging ability. Ferric ion reducing capacity was found to be higher in dry heated samples as compared to pressure cooked and boiled samples. Higher antioxidant activity of dry heated broad beans might be due to the formation of products from Maillard reaction. Tsai and She (2006) concluded that there was a change in the phenolic compounds after heating which resulted in increase in reducing power. The decrease in reducing power of pressure cooked samples correlates with the low level of phenolic contents since, during cooking, a part of phenolics diffuse from the seed coat to cooking water (Rocha-Guzman et al., 2007). The results of the study show that Fe chelating activity was higher in raw and dry heated samples. Similar results have also been reported in the raw and processed legumes of Macrotyloma uniflorum and D. lablab methanol and acetone extracts (Siddhuraju et al., 2008). The extract of peanut seed testa (Yen et al., 2005) and faba bean (Carbonaro et al., 1996) also showed a significant Fe2+ chelating effect.
CONCLUSION
The raw and dry heated samples of broad beans showed higher antioxidant activity than the pressure cooked and boiled samples. The results indicated that not only the phenolic constituent from raw samples but also the phenolics and Maillard products of processed samples are found to be potent antioxidant suppliers. Therefore, consumers may obtain optimal health benefits along with nutrient assimilation without any negative implications. As dry beans contain compounds other than phenolics that may have significant antioxidant potential, it will be useful to investigate their potential and maximize their use in food industry.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. A. Djeridane, M. Yousfi, B. Nadjemi, D. Boutassouna, P. Stocker, N. Vidal, Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds, Food Chem. 97 (2006) 654–660.
2. A.O.A.C. Official methods of analysis, Association of Official Analytical Chemists International. Maryland, USA, 2005.
3. B.D. Oomah, A. Cardador-Martinez, G. Loarca-Piña, Phenolics and antioxidative activities in common beans (Phaseolus vulgaris L), J Sci Food Agric, 85 (2005) 935–942.
4. B.J. Xu, S.H. Yuan, S.K.C. Chang, Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes, J Food Sci .72(2007)167–177.
5. C. M. Ying, A. Azlan, S. Hasan Al-Sheraji, F. A. Hassan and K. N. Prasad, Antioxidant Activities and Total Phenolic Content in Germinated and Non-Germinated Legume Extracts Following Alkaline-Acid Hydrolysis, Pak J Nutr, 12 (2013)1036-1041.
6. C.F. Chau, P.C.K. Cheung, Y.S. Wong, Hypocholesterolemic effects of protein concentrate from three Chinese indigenous legume seeds, J Agric Food Chem., 46(1998)3698–3701.
7. D. Kim, O. Chun, Y. Kim, H. Moon and C. Lee, Quantification of phenolics and their antioxidant capacity in fresh plums, J. Agric. Food Chem., 51(2003) 6509-6515
8. E.A. Komolafe and V.S. Obayanju, Principle of Food Processing and Preservation. 1st Edn., Double Birth Publishers, USA., (2003) 87.
9. F. Yamaguchi, T. Ariga, Y. Yoshirmura, K. Nakazaw, Antioxidative and antiglycation activity of garcinol from Garcinia indica fruit rind, J Agric Food Chem., 48 (2000) 180–185.
10. F.C. Barroga, A.C. Laurena, E.M.T. Mendosa, Polyphenols in mung bean (Vigna radiata (L.) Wilczek): determination and removal. J Agri and Food Chem, 33 (1985) 1006-1009
11. G. Yaciuk, and J. Sofose, Food drying proceeding of a not shop held at Edmonton Albeta, 6th-9th July (1981)
12. H. H.F. Koolen, da Silva M.A. Felipe, F. C. Gozzo, A. Q.L. de Souza, A. D.L. de Souza. 2013, Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa L. f.) by UPLC–ESI-MS/MS, Food Res Int, 51 (2013) 467–473.
13. K. Shimada, K. Fujikawa, N.T. Yahara, Antioxidative properties of xanthin on autoxidation of soybeanoil in cyclodextrin emulsion, J Agric Food Chem., 40(1992) 945–948.
14. M. Carbonaro, F.Virgili, E. Carnovale, Evidence for protein tannin interaction in legumes: Implications in the antioxidant properties of faba bean tannins, LWT Food Sci Technol., 29(1996)743–750.
15. M. Oyaizu, Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine, Japanese J Nutr. 44 (1986) 307–315.
16. N.E. Rocha-Guzman, R.F. Gonzalez-Laredo, F.J. Ibarra-Perez, C.A. Nava- Berumen, J.F. Gallegos-Infante, Effect of pressure cooking on the antioxidant activity of extracts from three common bean (Phaseolus vulgaris L.) cultivars, Food Chem. 10(2007) 31–35.
17. O.L. Milo, Nutraceuticals and functional foods. Food Technology, 58 (2004) 65–68.
18. P. Saini and P. Singh, Antioxidant activity and antimicrobial property of some Indian Spices, Trends in Biosci, 8(19) (2015) 5261-5267.
19. P. Siddhuraju and K. Becker, Studies on antioxidant activities of mucuna seed (Mucunapruriens var. utilis) extracts and certain non-protein amino/ imino acids through in vitro models, J Sci Food Agric., 83 (2003) 1517–1524.
20. P. Siddhuraju, The antioxidant of phenolic compounds extracted from defatted raw and dry heated Tamarindus indica seed coat. Lebensmittel Wis- senschaft und Technologie, 40 (2007) 982-90
21. P. Siddhuraju, V. Maheshu, N. Loganayaki, S. Manian, Antioxidant activity and free radical scavenging capacity of dietary phenolic extracts from processed indigenous legumes, Macrotyloma uniflorum (Lam.) Verdc. and Dolichos lablab, L. Food. 2 (2008) 159–167.
22. P.J. Tsai, C.H. She, Significance of phenol-protein interactions in modifying the antioxidant capacity of peas, J Agric Food Chem. 54 (2006)8491–8494.
23. R. Amarowicz, M. Karamac, F. Shahidi, Antioxidant activity of phenolic fractions of lentils (Lens culinaris), J Food Lipids, 10(2003)1–10.
24. R. Fernandez-Orozco, H. Zielinski, M.K. Pisku?a, Contribution of low-molecular-weight antioxidants to the antioxidant capacity of raw and processed lentil seeds, Nahrung Food. 47(2003) 291–299.
25. S. Ranganna. Handbook of Analysis and Quality Control for Fruit and Vegetable Products, Tata McGraw-Hill Education, 2005.
26. S.K. Yadav, A. Sehgal, Effect of home processing on ascorbic acid and beta-carotene content of spinach (Spinachia oleracia) and amaranth (Amaranthus tricolor) leaves, Plant Foods for Human Nutrition. 47 (1995) 125–131.
27. S.K. Yadav, A. Sehgal, Effect of home processing on ascorbic acid and beta-carotene content of bathua (Chenopodium album) and fenugreek (Trigonella foenumgraecum) leaves, Plant Foods for Human Nutr,50 (1997) 239–247.
28. U. Krings and R.G. Berger. Antioxidant activity of some roasted foods, Food Chem, 72 (2001) 223-229.
29. V. Maheshu, D.T. Priyadarsini, D. Teepica and J.M. Sasikumar, Effects of processing conditions on the stability of polyphenolic contents and antioxidant capacity of Dolichos lablab L, J Food Sci Technol., 50(4) (2013) 731–738.
30. V. Pascharicha, G. Satpathy, R. K. Gupta, Phytochemical and Antioxidant activity of underutilized legume Vicia faba seeds and formulation of its fortified biscuits, J Pharma and Phytochem, 3 (2014) 75-80.
31. W. Brand-Williams, M.E. Cuvelier, C. Berset, Use of a free radical method to evaluate antioxidant activity, LWT - Food Sci and Technol 28(1995) 25-30.
32. W.J. Yen, L.W. Chang, P.D Duh, Antioxidant activity of peanut seed testa and its antioxidative component, ethyl protocatechuate, LWT Food Sci Technol, 38 (2005) 193–200.
33. X.Y. Ye, H.X. Wang, T.B.G, Dolichin, a new chitinase-like antifungal protein isolated from field beans (Dolichos lablab), Biochem Biophys Res Commun., 269 (2000) 155–159.
34. Y. S. Velioglu, G. Mazza , L. Gao, B. D. Oomah, Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products, Journal of Agricultural and Food Chemistry. 46 (1998) 4113–4117.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License