IJCRR - 2(4), April, 2010
Pages: 03-19
Print Article
Download XML Download PDF
REVERSE PHASE - LIQUID CHROMATOGRAPHIC METHOD FOR THE ANALYSIS OF CLOPIDOGREL IN PHARMACEUTICAL DOSAGE FORM
Author: Tarun Jain*, Anil Bhandari, Sanjay Sharma, Veerma Ram, Laik Ahmed, Balaram Deora
Category: Healthcare
Abstract:A selective and sensitive reverse phase high-performance liquid chromatographic (RP-HPLC) method has been developed for the analysis of Clopidogrel in pharmaceutical dosage form. The elution of Clopidogrel and Diazepam (Internal Standard) was achieved using octadecyl C 18 column (4.6 i.d. / 5\? particle size/
250 mm length) as stationary phase. The mobile phase consisted of Methanol: Water {pH adjusted to 3.5 with 0.1% Acetic Acid} (95:05, v/v) as eluents at a flow rate of 1.0 mL min-1. Detection was carried out at 220 nm. Quantitation was done by external standard calibration method. The retention time of Diazepam (IS) and Clopidogrel bisulphate was found to be 3.39 and 4.41 minutes respectively. The method has been validated for linearity, specificity, robustness, accuracy and precision. Linearity for Clopidogrel bisulphate was
in the range of 10-100 \?g mL-1. The total run time of analysis was 6 minutes and the lower limits of detection (LOD) and Quantification (LLQ) were 0.020 and 0.050 \?g mL-1, respectively. This validated HPLC method using a simple mobile phase is sensitive enough for future monitoring Clopidogrel in biological samples.
Keywords: Clopidogrel; Reverse Phase –High Performance Liquid Chromatography; Therapeutic Drug Monitoring; Bioanalytical estimation of Clopidogrel.
Full Text:
1. Introduction:
Clopidogrel bisulphate [methyl (s)-2- chlorophenyl (4, 5, 6, 7-tetrahydrothieno- [3,2-C]pyridin-5-yl) acetate bisulphate](Figure 1) is an oral antiplatelet agent (thienopyridine class) to inhibit blood clots in coronary artery disease, peripheral vascular disease, and cerebrovascular disease. Clopidogrel is a pro-drug whose action may be related to adenosine diphosphate (ADP) receptor on platelet cell membranes. The specific subtype of ADP receptor that clopidogrel irreversibly inhibits is P2Y12 and is important in platelet aggregation and the cross-linking of platelets by fibrin. 1 The blockade of this receptor inhibits platelet aggregation by blocking activation of the glycoprotein IIb/IIIa pathway. The IIb/IIIa complex functions as a receptor mainly for fibrinogen and vitronectin but also for fibronectin and von Willebrand factor. Activation of this receptor complex is the "final common pathway" for platelet aggregation, and is important in the cross-linking of platelets by fibrin. Platelet inhibition can be demonstrated two hours after a single dose of oral clopidogrel, but the onset of action is slow, so that a loading-dose of 300- 600mg is usually administered. Clopidogrel is a pro-drug activated in the liver by cytochrome P450 enzymes, including CYP2C192 . The active metabolite has an elimination half-life of about 8 hours and acts by forming a disulfide bridge with the platelet ADP receptor 3, 4, 5 .
A few analytical methods including gas chromatography–mass spectrometry (GC–MS) 6 , liquid chromatography–mass spectrometry (LC–MS) 7, 8 and highperformance liquid chromatography (HPLC) with UV detection9, 10 have been published for determination of the clopidogrel in the biological fluids. In these methods however, complex two steps extraction methods6, 7 or long analytical run time (10 min7 , 16 min9 and 12 min10 ) are required. While LOQ of 0.1 µg mL-1 , 0.125µg mL-1 and 0.2 µg mL-1 have been reported in published papers8, 9, 10. In human single dose pharmacokinetic studies a LOQ of less than 0.03 µg mL-1 is required for measuring of the analyte up to three half lives post-dose. The present work describes simple and fast method for analysis of the Clopidogrel in pharmaceutical dosage formulation and biological fluids, with sensitivity of 0.05 µg mL-1 and analytical run time of 6.0 min.
2. Materials and Methods:
2.1. Chemicals and standard solutions:
Clopidogrel [purity 99.9%] was gifted by IPCA Pharmaceutical PVT Ltd, Ratlam [MP] and Diazepam [IS] from Alembic Pharmaceuticals, Baddi, Himachal Pradesh. All reagents used were of HPLC grade except acetic acid which was of analytical grade. Water was glass tripledistilled and further purified with a 0.44 µ filtration membrane using vacuum pump. A stock solution of Clopidogrel (200µg mL-1 ) and IS (100µg mL-1 ) was prepared in methanol. Working standards of the Clopidogrel (0.5–100 µg mL-1 ) were prepared by serial dilution of the stock solution in methanol. Working standard solution of the IS (10 µg mL-1 ) was prepared in methanol. All solutions were stored at 4 �C and found to be stable for at least 4 weeks.
2.2. Equipments:
The HPLC system used was Cecil ADEPT CE 4700 solvent delivery system, a system controller (CE 4900), a UV–PDA detector (CE 4200) operated at wavelength of 220 nm, a degasser and a data processor all from Cecil, England, United Kingdom.
2.3. Chromatographic conditions:
The method was developed using Thermo C18-ODS column [250mm×4.6mm I.D., 5µm particle size] which was protected by a guard column [1 cm X 4.0mm I.D, 5 µm particle size]. A mixture of consist of methanol and 0.1% acetic acid (pH adjusted to 3.5) [95:05 v/v] used as the mobile phase. The detection wavelength was 220 nm. The solutions were filtered, degassed and pumped at a flow rate of 1.0 mL/min. The injection volume was 20µl.
2.4. Preparation of sample solution:
Twenty Clopidogrel bisulfate (75 mg) tablets were weighed, transferred to a clean and dry mortar and ground into a fine powder. Tablet powder equivalent to 200mg drug was then transferred to a 100 ml volumetric flask, 100 ml of methanol was added, and the flask was attached to a vortex shaker for 10 min to disperse the material completely. Now the solution was centrifuged at 10,000 rpm for 10 min and supernatant was filtered through a 0.45µm pore size Nylon 66 membrane filter using vacuum pump. The filtrate was further diluted using mobile phase to give test solutions containing 1000 and 100µg mL-1 . The stock solution of pure Clopidogrel was prepared by dissolving 20 mg of pure drug in 100 ml of methanol to give stock solution of 200µg mL-1 . The solution was further diluted with mobile phase to prepare test solution of 100µg mL-1 .
2.5. Method validation:
The proposed method was validated as per ICH guidelines. [11]
2.5.1. Linearity and range:
A stock solution of the drug was prepared at strength of 200µg mL-1 . It was diluted to prepare solutions containing 10–100?µg mL-1 of the drug. The solution was injected in triplicate into the HPLC column, keeping the injection volume constant 20 µL.
2.5.2. Specificity and selectivity:
Specificity of the method towards the drug established through determination of purity for Clopidogrel peak after mixing and filtration with excipients. The resolution factor of the drug peak from the nearest other resolving peak was also studied.
2.5.3. Precision:
Solutions in triplicate, of three different concentrations (80, 100 and 120µg mL-1 ), were analyzed on the same day and the values of relative standard deviation (R.S.D.) were calculated to determine intra-day precision. These studies were repeated on three different days to determine inter-day precision.
2.5.4. Accuracy:
A known amount of standard drug was spiked in triplicate to the pre-analysed samples and the recovery of the drug was calculated. The accuracy of the method was calculated at three concentrations such as 80, 100 and 120 µg mL-1 . The assay concentrations of 80, 100 and 120 % were spiked to the pre-analysed samples.
2.5.5. Robustness:
Robustness of method had been tested by varying the following parameters like Column temperature (� 5 �C), alteration in wavelength (� 5λ), flow rate (� 10%), pH (� 0.2%) and variation in mobile phase.
2.5.6. LOD and LOQ:
To estimate the limits of detection (LOD) and quantitation (LLQ), diluted sample was injected six times and the signal-tonoise ratio (S/N) was determined. LOD and LLQ were regarded as the amounts for which S/N was 3:1 and 10:1 respectively.
2.6 Solution stability and system suitability parameters:
The solution stability of Clopidogrel in the assay method had been carried out by keeping the test solution of sample and pure drug in air-tight volumetric flask at room temperature for 48 hrs. The same solution was assayed for every 8 hr interval up to the study period of 48 hrs. The overall system suitability was evaluated for the proposed method. The system suitability parameter studied were peak asymmetry (10%), capacity factor, theoretical plates, % Relative standard deviation of peak area, LOD (ng mL-1 ) and LLQ (ng mL-1 ).
2.7. Analysis of Dosage Form:
Twenty Clopidogrel bisulfate [75 mg] tablets were weighed, transferred to a clean and dry mortar and ground into a fine powder. Tablet powder equivalent to 200mg drug was then transferred to a 100 ml volumetric flask. To this, 100 ml of methanol was added and vortexed for 10 min to disperse the material completely. This solution was then centrifuged at 10,000 rpm for 10 min and supernatant was filtered through a 0.45µm pore size Nylon 66 membrane filter and further diluted using mobile phase to give test solutions containing 100µg mL-1 . Six replicates of the final solution were analyzed and the observations were recorded. The above method was used for two different marketed formulations.
3. Results
3.1 Development and Optimization of the Stability Indicating Method:
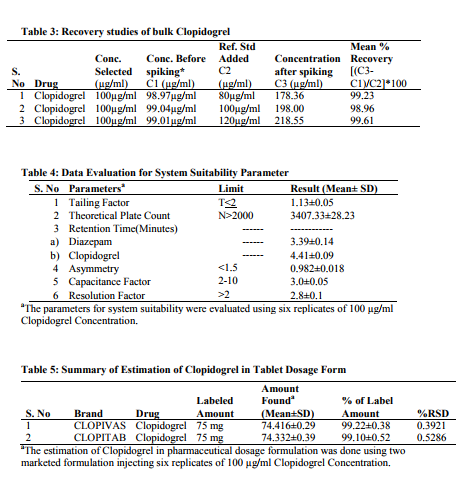
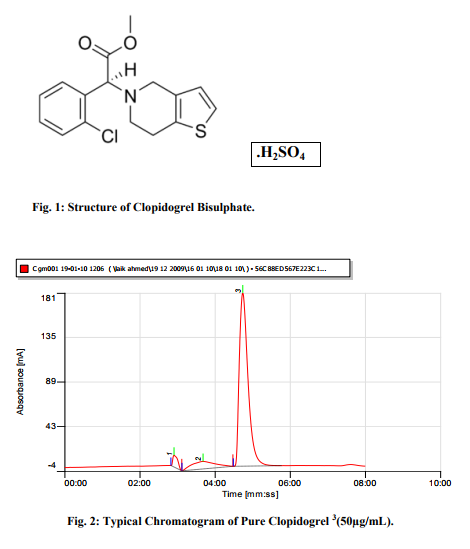
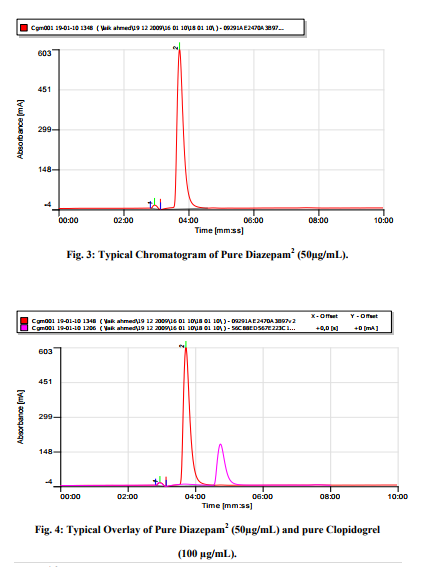
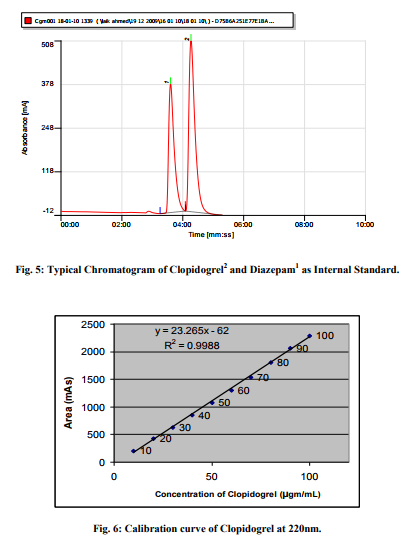
Satisfactory peaks were obtained with Methanol-Water (pH adjusted to 0.1% with acetic acid) at ratio 95:05, max 220 nm and flow rate 0f 1.0mL/min. The typical chromatogram under the optimum conditions is shown in figure 2 at Retention time 4.41 Minutes. The method has been optimized for future bioanalytical procedures using Diazepam as IS with retention time 3.39 Minutes as shown in figure 3. The overlay of Chromatograms shown in figure 4 indicates that the method succeeded in separation of drug and IS under similar set of conditions. A chromatogram of Clopidogrel with IS-Diazepam is shown in figure 5.
3.2 Analytical method validation:
The present study aims at the analysis of Clopidogrel in dosage formulation and its future application in biomedical analysis. The stability indicating assay methods are gaining importance for the evaluation of active pharmaceutical substance. United State Food and Drug Administration (USFDA) emphasize the stability indicating assay methods for the estimation of active ingredients. The analytical method has been validated through the determination of linearity, range, specificity, accuracy, precision, detection limits, qauntitation limits, ruggedness, robustness and system suitability parameters.
3.2.1 Linearity:
Linearity of the proposed method performed at concentration range of 10- 100µg mL-1 of Clopidogrel as shown in Table 1. The calibration curve drawn between concentration against the peak area counts shown in figure 6. The correlation coefficient (r2 ) found to be 0.9988. At 100�5 % of confidence limits, the regression equation is Y = 23.265X – 62..
3.2.2 Specificity:
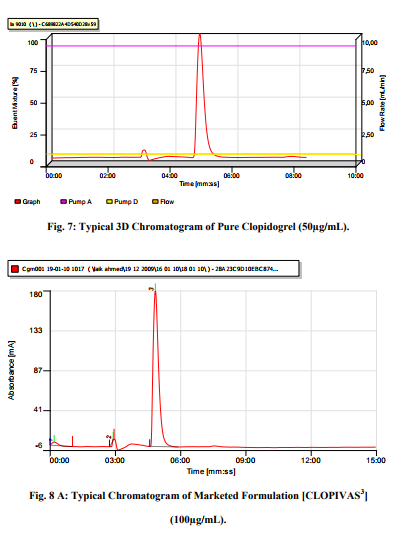
It is a measure of the degree of interference from such things as other active ingredients, excipients, impurities, and degradation products, ensuring that a peak response is due to a single component only i.e. that no co-elution exist. The mobile phase injected prior to the standard solution and the chromatogram of the diluent revealed that at particular conditions, there was no peak in the retention time of Clopidogrel. It showed that the method was specific. The 3D chromatogram of the stability sample shown in figure 7 demonstrated that the retention of Clopidogrel was unaffected by the other impurities.
3.3.3 Precision:
Precision of the proposed method was performed by intra and inter-day studies. Triplicates of three different concentrations (120, 100 and 80 µg mL-1 ) injected at 0, 2 and 4 Hours and on day 1, 2 and 3 for intra-day and inter-day precision respectively. The data obtained from precision experiments are shown in Table 2. The %R.S.D. values for intra-day and inter-day precision study were <0.5% and <0.7% respectively, confirming that the method was sufficiently precise.
3.3.4 Accuracy:
The accuracy of the method was studied at three concentrations such as 80, 100 and 120 µg mL-1 . The assay concentrations of 80 to 120 % were spiked to the respective pre-analysed samples and the recoveries were found to be 99.23, 98.96 and 99.61%. The accuracy of the method is shown in Table 3. Mean recovery (mean � S.D.) and relative standard deviation (%RSD) were found to be 99.26 � 0.326 and 0.328% respectively.
3.3.5 Robustness:
To evaluate method robustness a few parameters were deliberately varied like wavelength, pH, and flow rate. As evident from ANOVA statistical test, the calculated F value (0.768637) found to be less than the tabulated F value (4.06618) indicating that method is robust enough for the analysis of Clopidogrel within the specified range of deviation in the experimental conditions.
3.3.6 Ruggedness:
The ruggedness was found to be 0.94%. The acceptance criterion was 2 % and the outcomes of the experiments were within the limits.
3.3.7 Limit of Quantitation (LLQ) and Detection (LOD):
On the basis of signal to noise ratio, the LLQ and LOD found to be 20ng mL-1 and 50ng mL-1 respectively.
3.3.8 System suitability:
The overall system suitability was evaluated for the proposed method. The values of the chromatographic parameters were given in the Table 4.
3.4 Analysis of Pharmaceutical Dosage Formulation:
There was no interference from excipients used in the tablets. The drug content of the Tablet Formulation 1 and 2 were 99.22 � 0.38 (Mean � SD) with %RSD of 0.3921% and 99.10 � 0.52 (Mean � SD) with %RSD of 0.5286% respectively as shown in Table 5.
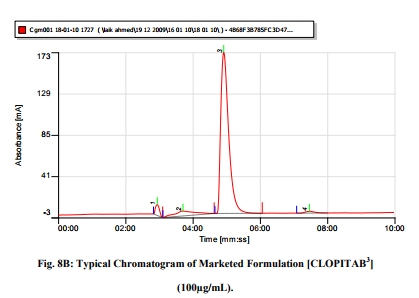
No degradation products were evident when commercial tablets where analyzed by this method as shown in figure 8-A [CLOPIVAS] and 8-B [CLOPITAB]. The good performance of the method indicates it to be suitable for routine analysis of Clopidogrel in pharmaceutical dosage forms. Further the LOD and LLQ of Method were sensitive enough to monitor the drug in biological fluids like plasma and urine.
4. Discussion:
4.1 Analytical method validation: Validated quantitative analytical methods that can detect the changes with time in the chemical, physical, or microbiological properties of the drug substance and drug product and that are specific so that the contents of active ingredient, degradation products and other components of interest can be accurately measured without interference. The chromatography techniques are profound and it has slighter edge over the other techniques because of the spectrum of various components in the sample matrix with the good accuracy. HPLC is widely used in the stability indicating assay methods. Due to the simplicity, selectivity, sensitivity and wider applicability; the Reverse Phase HPLC method with UV detector has been optimized and validated as per ICH guidelines. The accuracy of the method was performed by recovery studies. The recoveries at three different concentrations were found to be 99.23, 98.96 and 99.61%. The higher percentage mean recovery indicated that the validated method was accurate and reliable. The specificity of the method was confirmed by peak purity test of the stability samples, which proved that the spectral homogeneity of Clopidogrel peak was passed. The first derivative spectra of the standard and marketed samples showed that the Clopidogrel peaks resembled with respect to pattern, retention and resolution. The results using regression indicated that the method was linear in the concentration range of 10 to 100 µg mL-1 . As documented in the ICH guidelines, robustness should be considered early in the development of a method. As evident from ANOVA statistical test, the calculated F value (0.768637) is less than the tabulated F value (4.06618) indicating that method is robust for the analysis of Clopidogrel within the specified range of deviation in the experimental conditions. For Ruggedness, the acceptance criterion was 2 % and the outcomes of the experiments (0.94%) were within the limits, showed that the method was rugged.
The asymmetry of Clopidogrel was closed to 1.0 (0.982�0.018) which indicated that the peak shapes was symmetrical. The high counts of theoretical plates (3407.33�28.23) revealed the column efficiency. The capacity factor (3.0�0.05) indicated the selectivity of the method and the percentage RSD (1.67%) showed that the proposed method was precise.
5. Conclusion:
In this work, a quick, economic and sensitive method has been developed and validated. The retention time of IS and Clopidogrel were 3.29 and 4.41minutes respectively. The shorter run time favored the assay method for carrying out in-vitro release profile of drug. The LOD and LLQ were sensitive enough for analysis of samples in biological matrices such as blood, plasma and urine. This analytical method promises to be simple, economic, specific, and sensitive. The low amounts which can be quantitated with high accuracy and precision indicate that the method is sensitive, accurate and reliable. Therefore this method can be successfully applied for estimation of drug level in pharmaceutical formulations and monitoring of Clopidogrel in preclinical and clinical studies.
References:
1. Savi, P.; Zachayus, J.L.; Delesque, N.T. Proceedings of the National Academy of Sciences of the USA. 2006, 103 (29), 11069–11074.
2. Pereillo, J.M.; Maftouh, M.; Andrieu, A.; Uzabiaga, M.F.; Fedeli, O.; Savi, P.; Pascal, M.; Herbert, J.M.; Maffrand, J.P.; Picard, C. Drug Metab. Dispos. 2002, 30 (11), 1288–1295.
4. Simon, T. New Engl. J. Med. 2009, 360, 363–75.
5. Collet, J. Lancet. 2009, 373, 309
. 6. Lagorce, P.; Perez, Y.; Ortiz, J.; Necciari, J.; Bressolle, F. J. Chromatogr. B. 1998, 720, 107.
7. Ksycinska, H.; Rudzki, P.; Kiliszek, M.B. J. Pharm. Biomed. Anal. 2006, 41, 533.
8. Mitakos, I.; Panderi, I. Anal. Chim. Acta. 2004, 505, 107.
9. Singh, S.S.; Sharma, K.; Barot,D.; Mohan, P.R.; Lohray, V.B. J. Chromatogr. B. 2005, 821, 173.
10. Souri, E.; Jalalizadeh, H.; Kebriaee-Zadeh, A.; Shekarchi, M.; Dalvandi, A. Biomed Chromatogr. 2006, 20, 1309.
11. ICH, Validation of Analytical Procedures: Text and Methodology (Q2 (R1)), International Conference on Harmonization. IFPMA, Geneva, 2005.3. Mega, J. L. New Engl. J. Med. 2009, 360, 354–362.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License