IJCRR - 2(5), May, 2010
Pages: 30-41
Print Article
Download XML Download PDF
DESIGN AND EALUATION OF BACLOFEN MUCO ADHESIVE BUCCAL TABLETS
Author: Gavaskar Basani, B.Stephenrathinaraj, S.Sudharshini, A.Kishore reddy, Gajanan v.Shinde
Category: Healthcare
Abstract:The purpose of this research work was to establish mucoadhesive buccal tablets of Baclofen in the forms of monolayered tablets. The tablets were prepared using Sodium methyl cellulose (NaMC ), sodium alginate and Methocel K15M as bioadhesive polymers to impart mucoadhesion. Buccal tablets were evaluated by different parameters such as weight uniformity, content uniformity, thickness, hardness, surface pH, Swelling index, ex vivo mucoadhesive strength, in vitro drug release, and in vitro drug permeation. The present study concludes that mucoadhesive buccal tablets of Baclofen can be a good way to bypass the extensive hepatic firstpass metabolism and to improve the bio availability of Baclofen.
Keywords: Monolayered buccal Tablet, Buccal delivery, Mucoadhesion, Baclofen
Full Text:
Introduction
Amongst the various routes of drug delivery, oral route is perhaps the most preferred to the patient and the clinician alike. Accordingly, other absorptive mucosae are considered as potential sites for drug administration. Even though the rectal, vaginal, and ocular mucosae all offer certain advantages, the poor patient acceptability associated with these sites renders them reserved for local applications rather than systemic drug administration1 . Furthermore, oral transmucosal drug delivery bypasses first pass effect and avoids pre-systemic elimination in the GI tract. These factors make the oral mucosal cavity a very attractive and feasible site for systemic drug delivery. The precise mechanism of action of baclofen is not fully known. Baclofen is a muscle relaxant and anti spastic. Although baclofen is an analog of the putative inhibitory neurotransmitter gamma-aminobutyric acid (GABA), there is no conclusive evidence that actions on GABA systems are involved in the production of its clinical effects. Oral bioavailability is approximately 70%. Baclofen was selected as a model drug for the investigation because its oral dose is low (10mg)2 . Baclofen is a weak base and its pKa value is around 5, which satisfies the criterion for the selection of the drug. The log PC (partition coefficient) value for Baclofen is about 1.33. It indicates that Baclofen has sufficient lipophilicity to pass through the buccal membranes. The tmax of Baclofen is 1.0 hr by per oral route, which is long and variable3 . The dose of Baclofen is 20 mg twice a day, however, a lower effective dose is reported to be approximately 10 mg 4 . By observing the above points, it is inferred that Baclofen has a need to formulate into buccal tablets and the drug is suitable for it. A suitable buccal drug delivery system should be flexible and possess good bioadhesive properties, so that it can be retained in the oral cavity for the desired duration. In addition, it should release the drug in a predictable manner to elicit the required therapeutic response. In the present study, the objective was to prepare muoadhesive buccal tablets of Baclofen to prolong the residence time of the buccal tablets, which ensure satisfactory drug release to a mucosa and to avoid loss of drug resulting from wash out with saliva. The buccal tablets were evaluated by weight uniformity, thickness, hardness, surface pH, swelling index, ex vivo mucoadhesive strength, In vitro drug release and In vitro buccal permeation studies.
Materials and Method
Baclofen (99.96% purity), were gift samples from Cipla ltd .Pune. Carbopol 974p5 (Colorncon Asia Pvt ltd, Goa, India).
Methocel K15m6 (Colorncon Asia Pvt ltd, Goa, India).Sodium CMC7 (S.D.Fine chemicals Mumbai. India).PVP- K30. (S.D.Fine chemicals Mumbai. India).Sodium alginate. (S.D.Fine chemicals Mumbai. India)Lactose (Anhydrous) (Laxachem.organic Pvt ltdAmravati.India).Magnesium Stearate (Scientific India -Akola, India).Talc (Scientific India -Akola, India).All other reagents and chemicals used were of analytical reagent grade.
Preparation of Mucoadhesive Buccal tablets
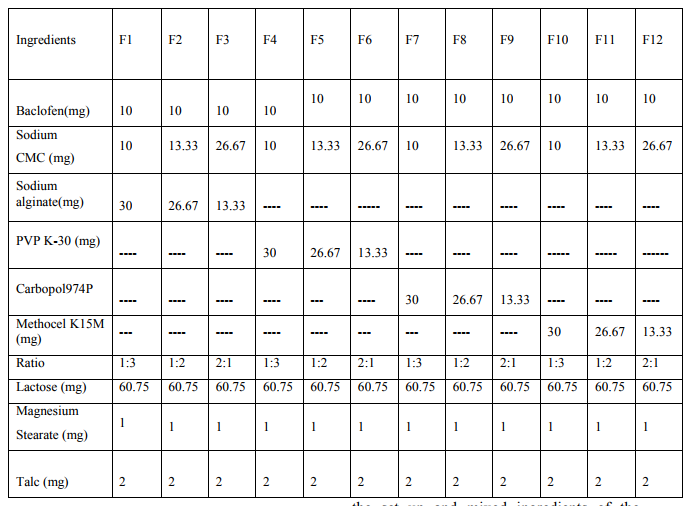
Mucoadhesive buccal tablets containing Baclofen were prepared by direct compression method. The ingredients of the core layer (Table 1) were accurately and mixed by trituration in a glass mortar and pestle. The mix was then compressed using 8 mm die by a tablet press. In order to obtain constant tablet weight the mannitol was added as filler exceipent in the core layer. After compression of tablet the upper punch was removed carefully with out disturbing the set up and mixed ingredients of the
Table No. 1: - Composition of Baclofen mucoadhesive buccal tablets. backing layer (Table1) were added over the tablet and compressed again
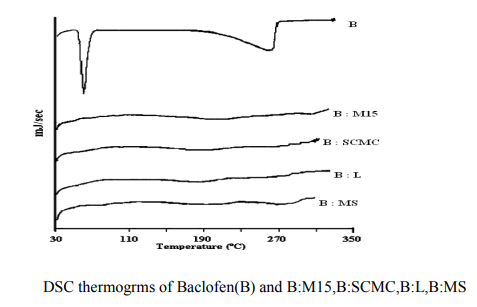
DSC Thermograms of Films
DSC thermograms of the Baclofen and it containing Methocel K15 , Sodium CMC, Lactose, and Magnesium Stearate were recorded on a thermal analyzer (Shimadzu DT- 40, Kyoto, Japan). The samples were heated from 30 to 300 C at a heating rate of 10 C/min in an inert nitrogen atmosphere.
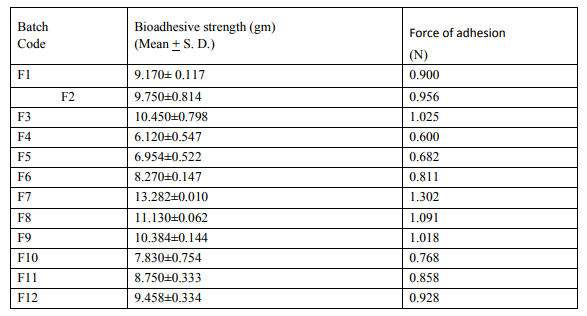
Ex vivo Mucoadhesive strength8-15
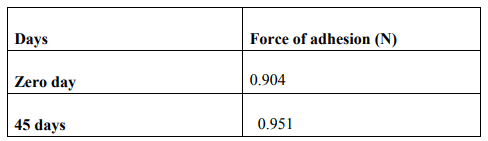
A modified balance method was used for determining the ex vivo mucoadhesive strength. Fresh sheep buccal mucosa was obtained from a local slaughterhouse and used within 2 hours of slaughter .The mucosal membrane was separated by removing underlying fat and loose tissues. The membrane was washed with distilled water and then with phosphate buffer pH 6.8 saliva solutions at 37o c.The Sheep buccal mucosa was cut into pieces and washed with phosphate buffer pH6.8. A piece of buccal mucosa was tied to the glass vial, which was filled with phosphate buffer. The glass vial was tightly fitted into a glass beaker (filled with phosphate buffer pH6.8 at 37o c+1 0 c) so that it just touched the mucosal surface. The buccal tablet was stuck to the lower side of a rubber stopper with cyanocarylate adhesive. The two sides of the balance were made equal before the study, by keeping a 5-g weight on the right-hand pan. A weight of 5 g was removed from the right hand pan. Which lowered the pan along with the tablet over the mucosa. The balance was kept in this position for 5 minutes contact time. The water (equivalent to weight) was added slowly with an infusion set (100 drops/min) to the right-hand pan until the tablet detached from the mucosal surface. This detachment force gave the mucoadhesive strength of then buccal tablet in grams (Table: 4).
Table No.4: - In-vitro Mucoadhesive strength study of prepared mucoadhesive buccal tablets of Baclofen.
Swelling Study15 The swelling study of tablets was determined by gravimetry.The swelling rate of the bioadhesive tablet was evaluated by using 1% agar gel plate (Table: 3). The average weight of the tablet was calculated (w1).The tablets were placed on gel surface in a Petri dish placed in an incubator at 37.10 c.Tablets was removed at different time intervals (1,2,3,4,5,6), wiped with filter paper and reweighed (w2).The swelling index was calculated by the formula. Swelling index = (w2. w1)/ w.
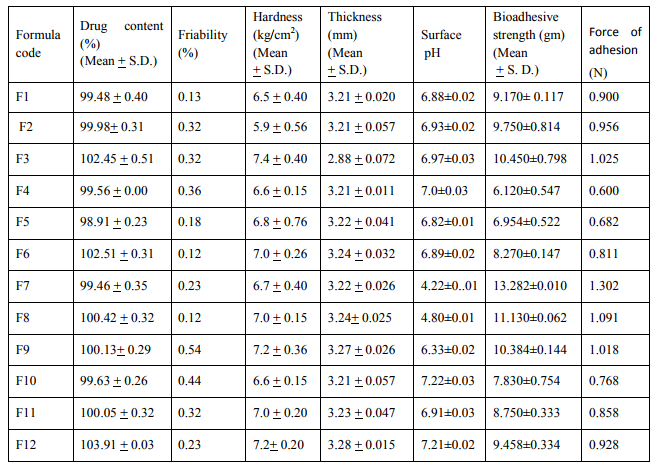
Surface pH study16,17
The surface pH of the buccal tablets was determined in order to investigate the possibility of any side effects in vivo. As an acidic or alkaline pH may cause irritation to the buccal mucosa, it was determined to using 1% agar gel plate (Table: 3). The average weight of the tablet was calculated (w1).The tablets were placed on gel surface in a Petri dish placed in an incubator at keep the surface pH as close to neutral as possible, The method adopted by Bottenberg et al used to determine the surface pH of the tablet. A combined glass electrode was used for this purpose (Table: 2). The tablet was allowed to swell by keeping it in contact with 1 ml of distilled water (pH 6.8 + 0.01) for 2 hours at room temperature. The pH was measured by bringing the electrode in contact with the surface of the tablet and allowing it to equilibrate for 1 minute.
Table no:2 Physico chemical properties of monolayer buccal tablet of Baclofen18-20.
In vitro dissolution studies21
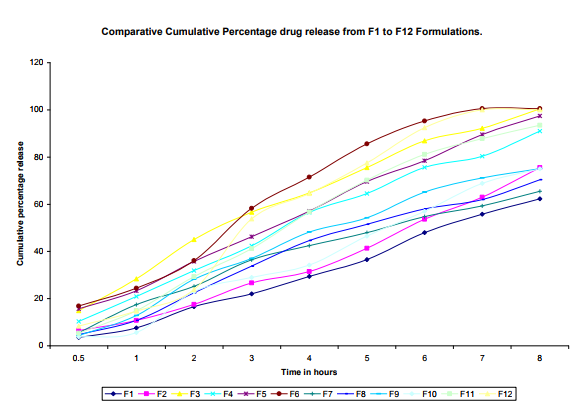
In vitro release study of mucoadhesive buccal tablets of Baclofen was carried out using the USP II (paddle apparatus) method at 50 rpm. Medium used for release rate study was 500 ml of phosphate buffer (pH 6.8) solution containing 20% methanol for maintaining the sink conditions. During the course of study whole assembly was maintained at 370 c. 2 ml of sample was withdrawn at specific time interval and add same amount of phosphate buffer after with drawing 2ml of sample its dilute with phosphate buffer(pH 6.8) up to 10 ml . Then amount of Baclofen released was determined spectroscopically at 223nm22 (Figure :1)
Ex vivo Diffusion study23
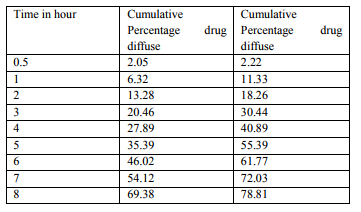
Ex-vivo diffusion study of pure drug was carried out using fresh porcine oral mucosa tissue, which was procured from local slaughterhouse and placed in Krebs buffer pH 7.4. Isolation of the epithelium was done mechanically by using scissors and forceps. These studied were carried out using open end cylinder method .The mucous membrane was tied to one end of the two sided open ended cylinder, which acted as donor compartment. The buccal tablet containing 6.25mg of Baclofen was kept on the mucous membrane, in such a way that the lower surface of the tablet was in contact with the mucous membrane Then the donor compartment was fixed, so that mucous membrane was in contact with the receptor medium(Beaker), 100ml of phosphate buffer, (PH 6.8) in the receptor compartment. Samples of 2 ml were withdrawn at periodic intervals from the receptor compartment and replaced with the fresh phosphate buffer immediately and after suitable dilution the drug content was analyzed spectrophotometrically at 223nm against a blank.
In vivo drug diffusion study23
A healthy rabbit weight 2.5 to 3 kg was taken and checked for absence of any disease. The fore limbs and hind limbs were fixed, into the iron rod of mini operation table so rabbit was not dorsal position. The prepared tablet was placed in a buccal membrane (Cheek pouch) with the help of forceps. Dextrose solution was given continuously throughout the period of study. Periodically 5 ml of blood sample were taken using a syringe which contained 1ml of 3.8% sodium citrate solution to prevent blood clotting. This blood sample subjected for centrifuging at 2200 rpm for 20 minutes.2ml of supernatant liquid was taken from this and a suitable dilution (10ml by buffer solution) and sample is analyzed at 223 nm in UV-spectrophotometer. The drug content obtained was subtracted from initial drug content in buccal tablet. The value obtained is denotes amount of drug release from buccal mucosa of rabbits. (Table: 5)
Stability Protocol
Stability studies were carried out on the formulation tablets of batch were first wrapped in aluminum foil then placed in an amber colored bottle. It stored at 40o c for 45days.Tablet was evaluated for physical characteristics; mucoadhesive properties, and in vitro drug release and muco adhesive strength study after 45days. Results obtained were compared with data obtained for zero time at ambient temperature. (Table :7)
Results and Discussion
In the present work efforts have been made to develop mucoadhesive buccal tablets of Baclofen using direct compression techniques involving mucoadhesive polymers like carbopol, various cellulose ethers having different degree of solubility and swelling ability, such as Sodium carboxy methyl cellulose. Hydroxy Propyl Methylcellulose was selected in oral products, it primarily used table binder and extended release matrix. Polyvinylpyrrolidone (PVP-K 30) was included as tablet binder. The FTIR spectral analysis showed that there was no appearance or disappearance of any characteristic peak, which confirms the absence of chemical interaction between drug and polymers. The bulk density and tapped density for Sodium CMC, Sodium alginate, PVP K 30, Carbopol 974P, Methocel K15M, was found to be 0.357 + 0.011 gm/ml, 0.442 + 0.0131 gm/ml, 0.256 + 0.019 gm/ml 0.381 + 0.129 gm/ml and 0.312 + 0.005 gm/ml, 0.409 + 0.331 gm/ml, 0.263 + 0.003 gm/ml, 0.381+0.321 gm/ml 0.333 + 0.015, 0.416 + 0.0125 gm/ml respectively. The bulk density and tapped density for Baclofen was found to be 0.277 + 0.02 and 0.326 + 0.051 gm/ml respectively24 The blend of ingredients was analyzed for physical characteristics. The angle of repose of compressibility index values for all polymers was found between 19 to 32 % and Hausner ratio above 1.23 to 1.47. Compressibility index value of sodium alginate and carbopol 974P is 32.39 + 1.86, 30.42 + 0.064 indicate poor. It conclude that the potential strength of polymer particle or not satisfactory for compression which also supported by lower density value and hausner ratio above 1.25.It reveals that all the formulation blends were having good flow characteristics and flow rates. All the formulation passes the test for weight variation as per the IP standard + 7.5% deviation. Percentage of drug content for all formulations F1 to F12 were in the range of 98.91 + 0.23 to 103.91 + 0.03. Thickness, Hardness of F1 to F12 formulations were found to be 2.88 + 0.072 to 3.28 + 0.015, and 5.9 + 0.56 to 7.4 + 0.40 The bioadhesive and drug release profile are dependent upon swelling behavior of the tablets. Swelling index was calculated with respect to time. The swelling index was for all formulations F1 to F12 (After 6 hours) were in the range 53.14-0.035 to 62.54-0.071. Buccal tablets of all the formulations surface pH values in the range of 4.22 0.01 to 7.22 0.03 that indicates no risk of mucosal damage or irritation. The bio adhesives property tablets of Baclofen containing varying proportions of polymers was determined with an insight to develop the tablets with adequate bioadhesiveness.The highest adhesion force and highest strength of the mucoadhesive bond was observed with the formulation as followed by F7 to F8. Tablets of formulation F4 to F5 containing showed least adhesion force than tablet of all other formulation. The batch F10 and F11 shows 74.98%, 93.50% of drug release which contain,1:3,1:2 ratio of sodium CMC and methocel K15M.The batch F11 shows promising result by releasing 93.50% drug release by controlled manner at 8 hours. This is due to the methocel K15M along with sodium CMC absorbed water rapidly with maximum swelling at 4 hours and start to welling erosion and finally drug release of at constant manner by zero order kinetic and swelling gel diffusion mechanism by applying zero order regression and peppas value of regression is correlated with each other. The formulation F11 showed 93.50 + 1.10 percentage of drug released at 8 th hr with good swelling index and bioadhesion strength. The optimized batch F11 shows dissolution zero order regression value and regression value of peppas plot as 0.9924,0.9933 is best correlated with each other also this values for Ex-vivo diffusion follows 0.9972,0.9959 and for In-vivo diffusion its value was 0.9982,0.9963 also indicate that it follows zero order kinetic along with swelling gel diffusion mechanism. The optimized batch F11 further subjected for in-vivo diffusion study by using rabbit. The result indicate that 69.38% of drug diffuse through rabbit buccal mucosa over the 8 hour study period also correlated exvivo diffusion and in-vitro dissolution. The ex-vivo diffusion study of Baclofen using porcine mucosa shows the almost 78.81% drug was permeated through the mucosa in 8 hrs.Results of stability studies of formulation F11 indicate that it is stable at 400C, for 45 days. There was no significant difference observed for dissolution and bioadhesion data.
Conclusion
The mucoadhesive buccal tablets of Baclofen may be a good way to by pass the extensive hepatic first-pass metabolism and to improve the bioavailability of Baclofen through buccal mucosa.
References:
1. Clarke E.C.G., Isolation and Identification of Drugs, the Pharmaceutical Press, London, 238, (1969).
2. Davidoff RA. Antispasticity drugs: Mechanisms of action. Ann Neurol 1985;17:107-16.
3. Meythaler JM, DeVivo MJ, Hadley M. Prospective Study on the use of bolus intrathecal baclofen for spastic hypertonia due to acquired brain injury. Arch Phys Med Rehabil 1996;77:461-6
4. Hulme A, MacLennan WJ, Ritchie RT, John VA, Shotton PA. Baclofen in the elderly stoke patient its sideeffects and pharmacokinetics. Eur J Clin Pharmacol 1985;29:467-9
5. Wade A. and Weller P. J., Handbook of Pharmaceutical Excipients, (2nded.) American Pharmaceutical Association, Washington D. C. and Royal Pharmaceutical Press, 71-73, (1994).
6. Gupta, A., Garg, S., and Khar, R. K., "Mucoadhesive buccal drug delivery system - A review", Indian Drugs, 29(13), 586-593, (1992).
7. Wade A. and Weller P. J., Handbook of Pharmaceutical Excipients, (2nded.) American Pharmaceutical Association, Washington D. C. and Royal Pharmaceu-tical Press, 78-80, (1994).
8 Bankar, G. S., Anderson, N.R., Lachman, L., Lieberman, H. A, and Kanig, J. L. (eds), The Theory and
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License