IJCRR - 2(5), May, 2010
Pages: 14-19
Print Article
Download XML Download PDF
ABSORPTION CORRECTION METHOD FOR ESTIMATION OF MEFENAMIC ACID AND TRANEXAMIC ACID IN COMBINED TABLET DOSAGE FORM
Author: Popat B. Mohite, Shantaram G.Khanage, Vaidhun H. Bhaskar
Category: Healthcare
Abstract:A new, simple, accurate and sensitive UVspectrophotometric absorption correction method has been developed for simultaneous determination of Mefanamic acid and Tranexamic acid in combined pharmaceutical dosage form. The method is based upon determination of Mefanamic acid at 352.0 nm and Tranexamic acid at 284.8 nm, in aqueous methanol (20 % v/v). Mefanamic acid and Tranexamic acid at their respective λmax 352.0 nm and 284.8 nm shows linearity in the concentration range of 4-40 \?g/ml and 4-40 \?g/ml respectively. The method was validated statistically.
Keywords: Mefanamic acid and Tranexamic acid, absorption correction method, standard addition
Full Text:
Introduction
Mefenamic acid is a synthetic antiinflammatory agent. Chemically it is a 2[(2,3,- dimethyl- phenyl)amino]benzoic acid. It is clinically useful in the treatment of inflammation. It is official in Indian pharmacopoeia1 It is listed in The Merck Index and Martindale2,3 . Chemically Tranexamic acid is Cis-4(amino methyl) cyclohexane carboxylic acid .TX is used in treatment of mennorhegia and fibrinolysis.It is listed in The Merck Index and Martindale4 . Literature survey reveals that only RP-HPLC5 and few spectrophotometric6,7 were reported methods are reported for the determination of Mefenamic acid and Tranexamic acid in combined with other drugs or alone. Hence the objective of the work is to develop new spectrophotometric methods for its estimation in bulk and formulations with good accuracy, simplicity, precision and economy.
Material And Method
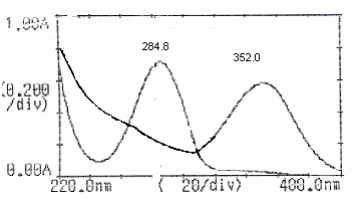
A UV/Visible double beam spectrophotometer, model Jasco V-630 with 1cm UV matched quartz cells was used. One Pan Balance (shimadzu) was used for experimental purpose. Methanol used was of analytical grade. M/s Blue Cross Ltd, Nashik provided the samples of MF and TX respectively. Mefenamic acid was standardized by official method reported in Indian Pharmacopoeia and the purity of the sample was found to be 99.75%. The purity of MF was 99.70%. Accurately weighed quantities (100 mg) of MF and TX were dissolved separately in 20 ml methanol and volumes were made up to 100 ml with water (1000 pg/ml). These solutions were used as working standards. Aliquot portions of stock solutions of MF and TX were diluted appropriately with aqueous methanol (20% v/v) to obtain concentration 20 pg/ml of MF and 20pg/ml of TX. The working standard solutions were scanned from 200 to 400 nm to select the wavelengths for estimation. From the overlain spectrum shown in Fig.1, the wavelength selected for estimation of MF was 352.0 nm, where TX has no absorbance and for MF it was 352.0 nm, where absorbance of TX is corrected. Different binary mixture solutions of MF and TX were then run in entire range from 200 to 400 nm. The drug obey Beer"s law in the concentration range of 0 to 35 pg/ml. Quantitative estimation of these drugs was carried out by using following formulae"s. Cy= A 284.8 nm / A (1%, 1cm) 352.0 nm of TX. (1) Cx = CAx 284.8 nm / A (1%, 1cm) 352.0 nm of MF .(2) CAx 352.0 nm = A 352.0 nm - Ay 284.8 nm(3) Ay 284.8 nm =Cy X A (1%, 1cm) 352.0 nm of TX (4) Where, Cx and Cy are concentration (mg /100ml) of MF and TX, respectively, A 284.8nm and A 352.0 nm are absorbance of mixture at 284.8 nm and 352.0 nm, respectively, CAx 352.0 nm is corrected absorbance of MF and Ay 284.8 nm absorbance of TX at 352.0 nm.
Fig.1: Over lay spectra of the both the drugs at selected wavelength
Assay of Tablet
For the estimation of the drug in tablet formulation twenty tablets were weighed and their average weight was determined. The tablets were then finely powdered.Appropriate quantity equivalent to 5 mg MF and 10 mg TX was accurately weighed and as per standard addition method, 9, 10, 20 mg of pure MF was added to achieve 2:1 ratio of MF and TX. The powder was transferred to 100 ml volumetric flask and shaken vigorously with aqueous methanol for 15 min and filtered. Necessary dilutions are made with aqueous methanol to give final concentration 20pg/ml and 20 pg/ml of MF and TX respectively. The absorbance?s values were read at 252.0 nm and 284.8 nm and concentration was obtained by solving the absorption correction equations. Results of analysis of tablet formulation are shown in Table No.
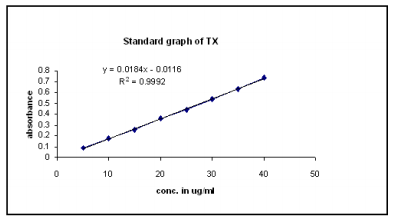
1.The proposed method was validated on the basis of parameters namely accuracy, precision, specificity, ruggedness and linearity and range. The accuracy of the proposed method was ascertained by carrying out recovery studies using standard addition method. The recovery study was performed to determine if there was any positive or negative interference from excipients present in the formulation. Precision of an analytical method is expressed as SD or RSD of a series of measurements. It was ascertained by replicate estimation of drug by the proposed method. The specificity is the ability to assess unequivocally the analyte for the presence of interesting components that may be expected to be present, such as impurities, degradation product and matrix components. During the specificity study, accurately weighed quantities of tablet powder equivalent to 10 mg of TX were taken in different 100 ml volumetric flasks. To them 20 mg of pure drug Nebivolol was added and stored for 24 hours under different conditions, like room temperature (normal), at 50 after addition of 1.0 ml of 0.1 N of hydrochloric acid , at 50 after the addition of 1.0 ml of 0.1 N of sodium hydroxide, at 50 after the addition of 3 % of hydrogen peroxide and at 60 . After 24 hours,the contents of the flask were subjected to same procedures as previously described. Test for ruggedness was carried out by repeating the procedure under different conditions, i.e., on different days, at different time and by different analysts. Linearity and range study was done by preparing concentration in the range of 80 - 120 % of test concentration and absorbance values were recorded at 352.0 nm and at 284.8 nm. The plot of linearity and range is shown in Fig. 2
Fig 2(a): Calibration curve for MF at 352.0 nm

Fig 2 (b): Calibration curve for TX at 284.8 nm
Results and discussion:
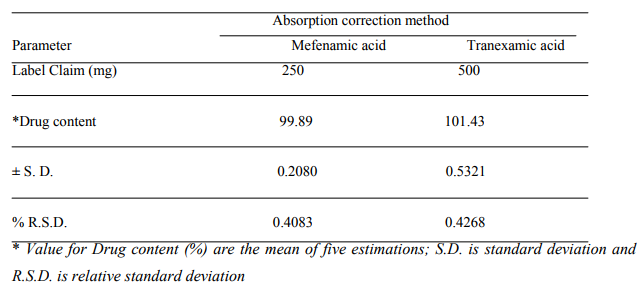
An attempt has been made to develop a fast, sensitive, precise, reproducible and economical analytical method for simultaneous estimation of MF and TX in their combined dosage form. In this method drugs obey Beer?s law in the concentration range of 5 to 40 pg/ml. The absorption additivity study was carried out to see whether the drugs in mixture showed additivity or not. It was observed that both the drugs showed additivity of absorbance at selected wavelengths indicating that both the drugs do not interact with each other in the solvent system used. A (1%, 1cm) values were also calculated for both the drugs. For MF, A (1%, 1cm) was found to be 130.4 0.8944 at wavelength 352.0 nm and for TX, it was 119 +0.7071, 354.4 0.8944 at wavelength 284.8 nm and 352.0 nm respectively. The result of percentage estimation of drug is shown in Table No.1
Table 1: Result of statistical analysis of the tablet dosage form.
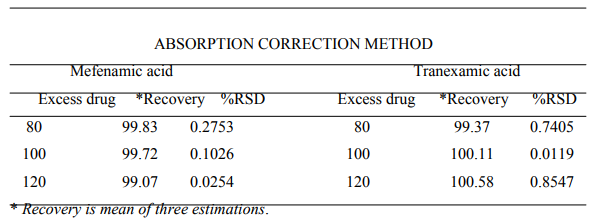
The method was validated as per the ICH and USP guidelines. The results of recovery study were found to be within the prescribed limit of 98 - 102 %, proving the accuracy and showing that the method is free from interference from excipients. The results are shown in Table No. 2.
Table No.2- Recovery studies data showing amount of drug recovered from sample
For precision, replicate estimation of both MF and TX in the same batch of tablets were done by proposed method, which yielded quite concurrent results, indicating reliability of the method. The values of SD or RSD are within the prescribed limit of 2 %, showing high precision of the method.. In the specificity study, the sample was exposed to different stress conditions like acid, alkali, Peroxide and heat. The study showed degradation of NBV under peroxide, but the exact nature of degradation of drug could not be found out. For ruggedness the proposed method was repeated under different conditions like different time, on different day and by different analyst. During the linearity study it was observed that absorbance values of MF and TX in the marketed formulation were linear in the range of 80 % to 120 % of the test concentration with R2 close to one for this method of analysis. From the study of validation parameters namely accuracy, precision ( SD and RSD), ruggedness (interday, intraday and different analyst), specificity, linearity and range, it was observed that the method is specific, accurate, precise,reproducible and rugged. Hence both the methods can beemployed for routine analysis of tablet dosage form.
Acknowledgements
We are highly thankful to Blue cross Lab. Ltd., Nashik for providing us the gift sample of the pure drug and to Principal M.E.S. College Pharmacy,Sonai for providing excellent research facilities.
References:
1. Indian Pharmacopoeia - 2007,Vol- 1,2,3,Govt Of India Ministry Of Health and Family Welfare, Published By: The Indian Pharmacopoeia Comision,Ghaziabad,Page No- 156,358,1468
2. Budavari S. The Merck index: An Encyclopedia of Chemicals, Drugs and Biologicals.13th ed. Whitehouse Station (NJ): Merck Research Lab, Division of Merck 2001.1152.
3. Budavari S. The Merck Index: An Encyclopedia of Chemicals, Drugs and Biologicals.13th ed. Whitehouse Station (NJ): Merck Research Lab. Division of Merck 2001: 854.
4. Sweetman SC, Eds., In; Martindale, The Complete Drug Reference, London; Pharmaceutical Press; Division of the Royal Pharmaceutical Society of Great Britain, 2007.875 (Electronic version. Available on http://www.medicinecomplete.com)
5. Dhake A.S. Indian Journal of Pharmaceutical science,volume- 49,no-3P.33-36.
6. Sadek E L M,Baraka M. Indian Journal of Pharmaceutical science,volume-58,No-5,1987.356 7. Basvaiah K.Indian drugs .vol-41,No- 5, May -2004,303.
8. ICH Q2A; Guidelines on validation of analytical procedure; Definitions and terminology, Federal Register, 1996,60.
9. ICH Q2B; Guidelines on validation of analytical procedure; Methadololgy, Federal Register, 1996, 60. 10. 27464. http:/www.xbl.com
11. Daharwal S J, Garg G, Saudagar R B, Saraf S (2006) Methods of estimation of multicomponent formulations. Indian J Pharma Sci 19(8):102.
12. Stenlake J B, Backett A H (1997) Practical pharmaceutical Chemistry, 4th Edition, Part 2, C. B. S. Publishers, p281.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License