IJCRR - 3(3), March, 2011
Pages: 81-87
Print Article
Download XML Download PDF
ANTIBACTERIAL PROPERTIES OF EXTRACTS IN CYANOBATERIA ISOLATED FROM COASTAL
REGION OF ORISSA
Author: Indira Priyadarshani, Hrudaynath Thatoi, Biswajit Rath
Category: General Sciences
Abstract:Biologically active substances were reported from cyanobacteria. Many strains of cyanobacteria
(blue green algae) are known to produce intracellular and extracellular secondary metabolites
with diverse biological activity such as antibacterial, antifungal, antiviral and antineoplastic
properties. In the present study attempt has been made to asses the antibacterial effect of aqueous
and methanol extracts of six marine cyanobacteria belonging to the genus Phormidium and
Lyngbya isolated from coastal region of Orissa against some Gram positive and Gram negative
human pathogenic bacteria following Disc diffusion assay method. The results showed that all the
test species of cyanobacteria exhibited antibacterial activity on aqueous and methanolic extracts.
However the intensity of antibacterial properties varied among the pathogenic strains of bacteria
used for the experimental purpose. Among the six cyanobacterial species the antibacterial effect
was found to be significant in Phormidium tenue and Lyngbya maintensiana as compared to other
test species. This shows greater biotechnological potential of these two species which needs
further studies.
Keywords: Aqueous, Cyanobacteria, Orissa coast, Secondary metobolites.
Full Text:
INTRODUCTION
Cyanobacteria are very old group of prokaryotic organism and relics of the oldest photoautotrophic vegetation in the World that occurs in fresh water, marine and terrestrial habitats. They vary from small single celled forms to complex multicellular forms. Cyanobacteria have drawn much attention as prospective and rich sources of biologically active constituent and have been identified as one of the most promising groups of organism to be able to produce bioactive compounds. Cyanobacteria occur in varied habitats ranging from marine to fresh water, from desert sand to hot springs and from snow field to ice caps. There are several reports of cyanobacterial compounds possessing a wide range of biological activity such as antibacterial, antiviral, antineoplastic effect ( Finical and Paul 1984, Hodgson 1984, Ballesteros et al., 1992, Bhosale et al., 2002). However the production capacity of antimicrobial substances by some species varies (Pesando, 1990). Screening of cyanobacteria for antibiotics and other pharmacologically active compound has received increasing interest as potential sources of new drugs ( Fish and Codd 1994, Borowitzka 1995, Ostensvik et al., 1998, Schlegel et al., 1999). The purpose of the present work was to evaluate the antibacterial activity of six strains of cyanobacteria viz. Phormidium tenue, Phormidium mole, Phormidium angustissium, Phormidium bohneri, Lyngbya maintensiana, Lyngbya chaetomorphae, against Gram positive bacteria, Staphylococcus epidermidis, Staphylococcus aureus, Bacillus brevis, Bacillus subtilis, Streptococcus aureus and Gram negative bacteria Escherichia coli and Shigella fleximinia. So far studies on biologically active substances from cyanobacteria isolates from Orissa coast has not been attempted. Hence the present study my provide valuable information for biological exploitation of cyanobacteria from Orissa coast for industrial applications.
MATERIALS AND METHODS
Algal samples were collected from different locations along Orissa coast during the month of March to June 2004 ( fig-1). Epiphytic and extraneous matters were removed by washing first in sea water and then in the fresh water. The samples were transported to the laboratory in the sterile polythene bags at ice temperature. The algal samples were separated into unialgal condition by repeated subculturing in enrichment media in both liquid and agar slants and were maintained at 26 ± 10C and 4000 lux light intensity with a photoperiod of 16h. light and 8h dark. Identification was done using morphological variation studies and taxonomical approaches according to Desikachary (1959). Altogether six species so far identified were taken as test organisms such as Phormidium tenue (Menegh) Gomont; Lyngbya maintensiana (Menegh) Gomont; Phormidium mole (Kutz) Gomont; Phormidium angustissium (W. et. G. S. West); Phormidium bohneri (Schmidle); Lyngbya chaetomorphae ( Iyengar et. Desikachary). The algal samples were shade dried, cut into small pieces and powdered in a mixture grinder. The extractions were carried out in solvents such as methanol and water (aqueous). These two extracts were tested for antimicrobial property on five Gram positive bacteria, Staphylococcus epidermidis (MTCC1114); Staphylococcus aureus (MTCC 25923); Bacillus bravis (MTCC 6853); Bacillus subtilis (MTCC 6633); Streptococcus aureus (MTCC3542), and two Gram negative bacteria, Escherichia coli (MTCC 11230) and Shigella fleximinia ( MTCC23223). The antibacterial assay was done following disc diffusion assay method (Casida 1986). 500µg of each extracts (50µl) dissolved in sterile filter paper disc (6mm) was used. After evapouration of the solvent the disc were placed in nutrient agar containing test pathogens. The plates were then incubated overnight at 370C and observations were made after 48h of incubation.
RESULTS AND DISCUSSION
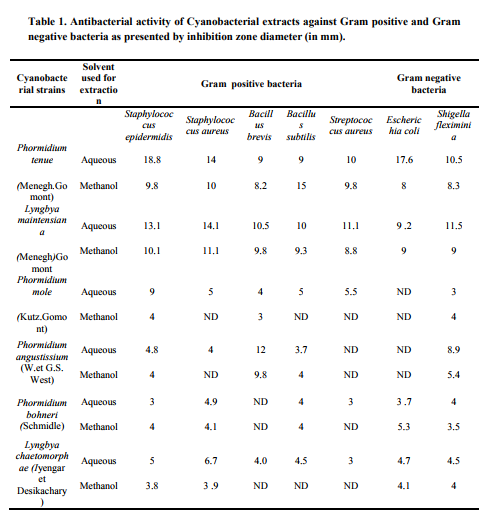
The results of antibacterial activity of six marine cyanobacteria isolated from coastal region of Orissa to different pathogenic strains of bacteria are represented in table-1. The result showed that all the test organisms possesses antibacterial property against all the strains of bacteria in both aqueous and methanolic extracts. However their intensity varies among strains basing on type of pathogenic bacteria and the extracts type. In this study it was observed that the test species of marine cyanobacteria Phormidium tenue and Lyngbya maintensiana showed significant antibacterial activity as compared to other four species. It is evident from the study that aqueous extracts of Phormidium tenue showed prominent antibacterial activity with inhibition zone size of 18.8mm diameter against Staphylococcus epidermis followed by inhibition zone of 17.6mm against E.coli. For other pathogenic strains of bacteria the size of inhibition zone ranges from 9 – 14mm. Compared to aqueous extracts, methanolic extracts showed size of inhibition zone ranging from 8-15mm against the test pathogenic Gram positive and Gram negative bacteria. Similarly Lyngbya maintensiana showed maximum size of inhibition zone i.e. 14.1mm against Staphylococcus aureus and minimum 9.2mm against E. coli in aqueous extracts. So far in Lyngbya maintensiana there was not much variations of antibacterial property between aqueous and methanolic extracts was observed. Among the test four species Phormidium mole and Phormidium angustissium showed moderate antibacterial activity in both aqueous and methanolic extracts which ranges from 3-9mm and 4- 12mm diameter size of zone of inhibition in these two species respectively. In other two species Phormidium bohneri and Lyngbya chaetomorphae the antibacterial property as expressed in zone of inhibition ranges between 3-5.3mm and 3- 6.7mm respectively. However in these four species the antibacterial property was not detected against few pathogenic test species of bacteria in aqueous and methanolic extracts as mentioned in the table-1. The results so far obtained was encouraging as all the strains of maine cyanobacteria isolated from coastal region of Orissa showed antibacterial activity, although the degree of antibacterial activity varies with species. Among the test species of cyanobacteria Phormidium tenue and Lyngbya maintensiana showed promising antibacterial activity against multiple strains of pathogenic bacteria in varied pattern in the aqueous and methanolic extracts. This variation might be due to masking of antibacterial activity by the presence of some inhibitory compounds or factors in the extracts as observed by Sastry and Rao, (1994). The antibacterial activity was found to be higher in aqueous extracts than the methanolic extracts, this is probably because of polar nature of active compounds. The differential antibacterial response of the test cyanobacteria to Gram positive and Gram negative group of bacteria may be attributed to the production of active compounds. The result of this work indicates that?s the marine cyanobacteria of Orissa coast which displays potential source of bioactive compounds which can be biotechnologically exploited for industrial application. Orissa coast extends over 480km along Bay of Bengal from Chandipur in the north to Gopalpur in the south with Puri coast at the centre. The samples were collected in these regions which are quite apart in their physiographic and environmental conditions and harbours different cyanobacterial strins suitable for growth in such habitats which needs further periodical study at different locations along the coastal region in this direction to reach at a conclusion.
CONCLUSION
Results from the present work indicate that the species of marine cyanobacteria from orissa coast examined showed a variety of antimicrobial activities and presence of bioactive molecules. Further isolation and identification of the active ingredients need to be done in order to understand their bioprospects. Since different activities were observed in extracts obtained with organic solvent and extracts obtained with water we can suggest that compounds with different polarities are involved. Thus the present work will contribute to an understanding that these bioactive compounds will need further studies to identify the chemical structure of these compounds and to examine their beneficial effect for inhibition of some pathogenic bacteria as antimicrobial metabolites of marine cyanobacteria are of special interest now in the development of new environment harmless.
ACKNOWLEDGEMENT
The authors thank Dr. N. Thajuddin, Dept. of Microbiology, Bharathidasan University, Tiruchirappalli (India) for identifying the cyanobacteria strains isolated from Orissa coast.
References:
1. Ballesteros, E.., Martin, D. and Uriz, M. J. (1992). Biological activity of extracts from some Mediterranean macrophytes. Bot Mar. 35:481-485.
2. Bhosale, S. H., Nagle, V. L. and Jagtap, T. G. (2002). Antifouling potential of some marine organisms from Indian species of Bacillus and Pseudomonas. Mar. Biotechnol., 4:111-118.
3. Borowizka, M. A. and Borowizka, L. J. (1988). Microalgal Biotechnology. Cambridge University Press, Cambridge, pp. 456-458.
4. Borowizka, M. A. (1995). Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol., 7:3-15.
5. Casida, I. E. (1986). Industrial Microbiology, Wiley Eastern Limited, New Delhi. pp. 106-107.
6. De Caire, G. Z., De Cano, M. M. S., De Mule, M. C. Z. and De Halperin, D. R. (1993). Screening of cyanobacterial bioactive compounds against human pathogens. Phyton. 54:59-65.
7. Desikachary, T. V. (1959). Cyanophyta. Indian Council of Agricultural Research New Delhi, New Delhi.
8. Fenical, W. and Paul, V. J., (1984). Antibiotic and cytotoxic terpenoids from tropical green algae of the family Udoteaceae. Hydrobiologia, 116/117:137- 140.
9. Falch, B. S., Konig, G. M., Wright, A. D., Sticher, O., Angerhofer, C. K., Pezzuto, J. M. and Bachmann, H. (1995). Biological activities of cyanobacteria: evalution of extracts and pure compounds. Planta Med., 61:321- 328.
10. Fish, S. A. and Codd, G. A. (1994). Bioactive compound production by thermophilic and thermo tolerant cyanobacteria (blue green algae). World J. Microb.Biotech., 10:338-347.
11. Flores, E. and Wolk, C. P. (1986). Production, by filamentous, nitrogen fixing cynobacteria, of a bacteriocin and of other antibiotics that kill related strains. Arch. Microbiol., 145:215-219.
12. Hodgson, L. M. (1984). Antimicrobial and antineoplastic activity in some south Florida seaweeds. Bot. Mar., 27:387- 390.
13. Jaki, B., Heilmann, J., Linden, A., Volger, B. and Sticher,O. (2000). Novel extra cellular diterpenoid with biological activity from the cyanobacterium Nostoc commune. J. Nat. Prod., 63:339- 343.
14. Jaki, B., Heilmann, J., Linden, A. and Sticher, O. (2000). New antibacterial metabolites from the cyanobecterium Nostoc commune (EAWAG 122b). J. Nat. Prod., 63:1283-85.
15. Ostensvik, O., Skulberg, O. M., Underal, B. and Hormazabal, V. (1998). Antibacterial properties of extracts from selected planktonic freshwater cyanobacteria, a comparative study of 87 International Journal of Current Research and Review www.ijcrr.com Vol. 03 issue 03 Mar 2011 bacterial bioassays. J.Appl. Microbiol., 84: 1117-24.
16. Patterson, G.M. L., Larsen, L. K. and Moore. R. E. (1994). Bioactive natural products from blue green algae. J. Appl. Phycol., 6:151-157.
17. Pesando, D. (1990). Antibacterial and antifungal activities of marine algae, In: I.Akatsuka (ed.) Introduction to Applied Phycology. SPB Academic Publishing, The Hague. pp. 3-26.
18. Pesando, D. and Caram, B. (1984). Screening of marine algae from the French Mediterranean coast for antibacterial and antifungal activity. Bot. Mar., 27:381-386.
19. Sastry, V. M. V. S. and Rao, G. R. K. (1994). Antibacterial substances from marine algae: successive extraction using benzene, chloroform and methanol. Bot Mar. 37: 357-360.
20. Schlegel, I., Doan, N. T., De Chazol, N. and Smith, G. D. (1999). Antibiotic activity of new cyanobacterial isolates from Australia and Asia against green algae and cyanobacteria. J.Appl. Phycol., 10:471-479.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License