IJCRR - 2(8), August, 2010
Pages: 07-24
Print Article
Download XML Download PDF
LOW MOLECULAR WEIGHT CHITOSAN AS A VEHICLE FOR SOLUBILIZATION AND AMORPHIZATION OF NON STEROID ANTI-INFLAMMATORY DRUG FOR A NEW GUAR GUM-BASED COLON DELIVERY FORMULATION
Author: Elkhodairy Kadria , Barakat Nahla, Alanazi Fars
Category: Healthcare
Abstract:The solubilization and amorphization of the poorly water soluble nonsteroidal antiinflammatory drug, Etodolac (ETD) with a hydrophilic polymer, low molecular weight chitosan (CHT), have investigated. Phase solubility studies were carried out to obtain an insight on the nature of a possible interaction between ETD and CHT in
solution. Binary systems of varying drug polymer ratios were prepared using different techniques namely physical mixing, cogrinding and kneading. Drug\?polymer interactions were investigated in solid stateby differential scanning calorimetry, powder X-ray diffractometry and scanning electron microscopy. The results obtained indicated loss of drug crystallinity. Dissolution rate studies revealed that the drug dissolution was improved with increasing the polymer concentration in the mixture in the following order kneading> co-grinding> physical mixing > pure drug. The prepared capsules containing the binary mixture prepared by
kneading in the drug-polymer ratio of 1:19 were coated with guar gum (a film coating material) and their dissolution rates were tested in comparison with commercial capsules present in the Egyptian market. A coating of 10 layers of guar gum prevented the release of etodolac in the acidic pH and permitted its release in colonic environment.
Keywords: Etodolac, Binary mixtures, Chitosan, Film coating, Guar gum.
Full Text:
Introduction
Oral colon-drug delivery system (CDDS) has been developed as one of the sitespecific drug delivery system. This delivery system, by means of combination of one or more controlled release mechanisms, hardly releases drug in the upper part of the gastrointestinal (GI) tract, but rapidly release drug in the colon following oral administration. The necessity and advantage of CDDS have been well recognized and reviewed [1]. In view of CDDS specifically delivering drug to the colon, a lot of benefits would be acquired in terms of improving safety and reducing toxicity when treating local or systemic chronic diseases, i.e. ulcerative colitis, Crohn?s disease, carcinoma and infections, the optimal colonic drug delivery system, should selectively deliver drug to the colon, but not to the upper GI tract (1). For this reason, the drug concentration was significantly lessened in the upper GI tract, while increased considerably in the colon, resulting in alleviated GI side effects [1]. In addition, CDDS would be advantageous when delay in absorption is desirable from a therapeutical point of view, as for the treatment of diseases that have peak symptoms in the early morning and that exhibit circadian rhythms, such as nocturnal asthma, angina and rheumatoid arthritis [2, 3]. The majority of colorectal cancers (CRCs) are thought to arise as the result of a series of molecular changes that transform normal colonic epithelial cells to adenomatous polyps and ultimately, to invasive cancers [4, 5]. The traditional non-steroidal antiinflammatory drugs (NSAIDs) and selective cyclooxygenase-2 (Cox-2) inhibitors potentially inhibited polyp development and tumour incidence [6]. Cox-2 over expression is thought to play an important role in colon carcinogenesis, as it has been found to be elevated in 40% of colonic adenomas and up to 90% of sporadic CRC [7, 8]. Its pharmacological inhibition by NSAIDs is the central event in the chemoprevention of colon cancer [6, 9-11]. A study showed that treatment with etodolac (ETD) significantly reduced the occurrence of neoplasia, suggesting that this Cox-2 inhibitor has chemopreventive activity against colitisassociated tumorigenesis [12]. Additionally, many researches emphasized the use of ETD for the treatment of prostate cancer [13]. Recent evidences indicated that Cox-2 selective inhibitors may also synergise with new chemotherapeutic agents [6]. It has been reported that ETD when combined with carboplatin, can enhance an action of the anticancer drug through the suppression of FAP-1 expression [14]. Long-term etodolac treatment effectively reduced metachronous cancer development in patients with extensive metaplastic gastritis [15]. Etodolac exhibited antitumor activity and induced E-cadherin expression in bladder cancer cells and might be useful for the clinical treatment and prevention of bladder cancer, especially in poorly differentiated bladder cancer with high COX-2 and low E-cadherin expression [16]. Therefore, many researches intended to formulate ETD as a colon drug delivery for the treatment of colorectal diseases as well as for the management of osteoarthritis [16]. Etodolac is a selective Cox-2 inhibitor, nonsteroidal anti-inflammatory drug. It is used in the management of osteoarthritis, postoperative pain, fever and inflammation. It is chemically designated as 2- (1, 8- Diethyl-4,9-dihydro-3H-pyrano[3,4-6] indol-1-yl) acetic acid. It is poorly soluble in water [17]. For poorly water soluble drugs, the rate of oral absorption is often controlled by the dissolution rate in the gastrointestinal tract. Therefore, the solubility and /or dissolution rate of a drug are key determinants of its oral bioavailability [18]. Thus, increasing the aqueous solubility and dissolution rate of ETD is of therapeutic importance. Several attempts have been made to improve the ETD dissolution properties via solid dispersion technique [19], complexation with cyclodextrin [20, 21] and selfemulsifying technique [22]. The present study was carried out to investigate the feasibility of chitosan enhancing the dissolution of ETD. Chitosan was selected because of its interesting properties, mainly oral biocompatibility, antiulcerogenic activity, direct compression property as well as its colon targeting potency [23]. Chitosan has been used to enhance the dissolution and bioavailability of a number of poorly water soluble drugs [24-27]. Low molecular weight chitosan can function as drug release enhancers for poorly water soluble drugs due to an improvement in wettability resulting from the solubility of low molecular weight chitosan in water [28]. The final goal of our work is to prepare a colon drug delivery system containing the chitosan: etodolac mixture that gave promising results using guar gum. Guar gum was selected as it is a specific colon drug delivery carrier as well as a free film former [29]. In vitro drug release studies have shown that guar gum in the form of compression coat applied over indomethacin core tablets protected the drug from being released under conditions mimicking mouth to colon transit [30] The same results were obtained when guar gum was used as a compression coat for metronidazole and 5- fluorouracil [31,32]. Based on these results, a trial was made to formulate coating solution of guar gum applicable for capsule coating. Solvent costs, environmental pollution and operator safety have driven the move from organic solvents to aqueous film coating. But the replacement of organic solvents has increased the complexity of process. So to take the advantage of both solvent and aqueous coating processes and overcome their limitations, a combined formulation was developed comprising the major portion of solvent as water and minor portion of solvent as organic solvent. This combination of solvents improves the drying rate which is the most critical parameter in aqueous film coating [33]. It has been reported that guar gum solution in a mixture of isopropanol and water in the ratio of 3:7 could be used as a film coating for tablets [33]. Capsules filled with the appropriate amount of the selected mixture of ETD/CHT were coated using guar gum solution with different coat thickness in an attempt to find out the optimized coat thickness that would inhibit ETD release in the acidic environment.
Materials and methods Materials
Etodolac (ETD) was kindly received from Pharco Pharmaceutical Company (Alexandria, Egypt). Chitosan low molecular weight (CHT) was supplied by Sigma Chem. Co. (St. Louis, USA). Guar gum was supplied by Merck Chem. Co. (Bombay, India). All other chemical were of analytical grade and used without further purification.
Methods Phase solubility studies
Aqueous solubility of ETD in presence of CHT was carried out according to the method described by Higuchi and Connors (34). An excess amount of ETD was added to 10 ml of aqueous solutions containing increasing concentration of CHT (0, 0.25, 0.5, 0.75, 1, 1.25, 1.5 and 2% w/v) in screwcapped vials. The suspensions were shaking in a thermostatically controlled water bath (GFL, type 1083, GmbH and Co, Burgweded, W. Germany) at 37 ± 0.5 C° for 48 hrs. After equilibrium has been attained (2 days), aliquots were withdrawn, filtered through 0.45 µm membrane filters, suitably diluted and analyzed for ETD using UV spectrophotometer at 279 nm. Preparation of binary systems ETD-CHT binary systems were prepared using varying drug concentration of 5, 10 and 20 % w/w equivalent to drug: polymer ratios of 1:19, 1:9 and 1:4, respectively. The binary systems were prepared using different methods. Physical mixtures (PMs) of drug and polymer were obtained by simply blending with spatula, co-ground (CG) by co-grinding of drug and CHT for 30 minutes in a ceramic mortar and kneaded mixtures were prepared by kneading the drug-CHT mixture with ethanol-water 6:1 v/v in a ceramic mortar followed by drying in oven for 48 hrs at 40 ºC. The powdered products were sieved (68-125 µm) and were used for subsequent studies. The samples were stored in desiccators till used. Drug content An amount of the prepared powders equivalent to 10 mg of ETD were weighed accurately and triturated with 10 ml of phosphate buffer pH 6.8 and finally the volume was made up to 100 ml with the buffer solution. The solution was filtered through a membrane (0.45 µm). The drug content was analyzed using the filtrate after suitable dilution at 279 nm using UV spectrophotometer (Perkin Elmer, USA). Each sample was analyzed in triplicate.
Particle Size Analysis
The size distribution of physical mixture, co-ground and kneading mixture was measured with a laser diffraction particle size analyzer (SALD-2101 Shimadzu, Japan). The particle size distribution and mean particle size diameter were automatically calculated using the software provided. The size distribution was evaluated with the span value defined as follows. Span = (D90% - D10%) /D50% Where, DN% (N= 10, 50, 90) means the volume percentage of microparticles with diameters up to DN%. The smaller span value indicates the narrower particle size distribution.
Scanning electron microscopy (SEM) The surface morphology of drug, chitosan, PM, CG and KM was examined by means of double sided adhesive tape was placed on an aluminum specimen holder upon which a small amount of powdered samples was deposited. The particles were coated with approximately 10-20 nm gold for 20 S using a sputter coater. Scans were performed at an acceleration voltage of 20 Kv (Jeol, JSM- 6360LV scanning microscope, Tokyo, Japan). The instrument was calibrated for temperature and heat flow using high purity indium and zinc standards.
Differential scanning calorimetry (DSC) DSC
thermograms of pure materials, PM, CG and KM were recorded using Shimadzu differential scanning calorimeter (TA 501 Shimadzu, Japan). Samples (2-4 mg) were placed in sealed aluminum pan was used as a reference. Scanning speed 10 oC min -1 , in the 25–200 oC temperature range. The equipment was periodically calibrated with indium.
X-ray diffractometry
The X-ray diffractograms of pure materials, PM, CG and KM were carried out using a Siemens diffractometer (Siemens D500, Germany) where Cu Kα, radiation was selected by a Ni monochromator. The scanning rate employed was 2°/min over a diffusion angle of 2θ and range of 5-60 °, operated at a voltage of 30 KV and a current of 30 mA, the scan step size was 0.018 (2θ). The analysis was carried out at room temperature under ambient conditions.
In-vitro dissolution study
ETO dissolution study was evaluated using the USP XXIV dissolution rate apparatus II (Pharmatest, Germany) at a stirring rate of 100 ± 2 rpm. Powders samples containing 100 mg of pure drug or its equivalent amount of PM, CG and KM were placed in 900 ml phosphate buffer pH 6.8 at 37± 0.5 ºC for 2 hrs. At predetermined time intervals, 5 ml samples were withdrawn and immediately replaced with an equal volume of prewarmed dissolution medium. All samples were run in triplicate, filtered through 0.45 µm membrane filter and the amount of dissolved ETO was analyzed by spectrophotometer at 272 nm. The In-vitro dissolution study ETO dissolution study was evaluated using the USP XXIV dissolution rate apparatus II (Pharmatest, Germany) at a stirring rate of 100 ± 2 rpm. Powders samples containing 100 mg of pure drug or its equivalent amount of PM, CG and KM were placed in 900 ml phosphate buffer pH 6.8 at 37± 0.5 ºC for 2 hrs. At predetermined time intervals, 5 ml samples were withdrawn and immediately replaced with an equal volume of prewarmed dissolution medium. All samples were run in triplicate, filtered through 0.45 µm membrane filter and the amount of dissolved ETO was analyzed by spectrophotometer at 272 nm. The
Preparation of coating solution
Preliminary experiments were carried out to obtain guar gum solution of appropriate characteristics of viscosity and homogeneity. Solutions of different ratios of water-isopropanol were prepared to find out the optimum ratio for the preparation of 1% w/w gum solution. . The experiment was designed to prepare ratios ranging in the order of: 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9. The weighed amount of guar gum was dissolved in the appropriate amount of water, and then the isopropanol was added dropwise till an almost clear solution was obtained. The solution was mixed thoroughly to effect homogeneity. It was found that the water: isopropanol ratio of 7:3 is the promising ratio for optimum solubility of the gum. This result was in agreement with the work done by Rane and Kale [33].
Preparation of guar-coated capsules
Capsules containing an amount of KM ( 5% w/w) equivalent to 100 mg of ETO was enteric coated with guar gum 1% w/v in mixture of water and isopropanol in the ratio of 7:3 [33]. The enteric coating solution was prepared by dissolving the calculated amount of guar gum in distilled water to prepare 1% w/v solution; Isopropyl alcohol was added drop wise to adjust the viscosity of the coating solution without any precipitation of gum. The prepared coating solution was stirred to effect homogeneity. Coating procedure by dipping method was adjusted to suit the requirements for aqueous coating [34]. For the first three coat the capsule was immersed in the coating solution for 5 seconds and the coating was dried by blowing hot air using hair dryer (drying time was 10-15 minutes), in the subsequent coating, the dipping time was increased to 10 seconds and drying time was reduced to 5-10 minutes. This manipulation in the coating process was essential to avoid the migration of water from coating solution into the capsule core. Capsules of different coat thickness (5, 8 and 10 coats) were prepared and subjected to dissolution studies.
Dissolution studies of coated capsules
The dissolution studies of the prepared coated capsules were performed as previously mentioned. The capsule dissolution study was performed for the first 2 hrs in pH 1.2 then the pH of the medium was render 6.8 by the addition of calculated amount of trisodium hydrogen phosphate. The dissolution study in pH 6.8 was carried out for further 3 hrs.
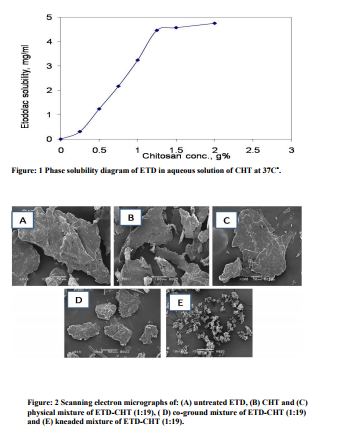
Results and discussion Phase solubility studies
In order to gain insight into the nature of a possible interaction between ETD and CHT in solution, phase solubility experiments were performed. The phase solubility profiles for the ETD- CHT systems were presented in Figure 1. The diagram showed that the aqueous solubility of the drug is increased linearly as a function of CHT concentration. The solubility curve was regarded as an AN type of phase solubility diagram. These results are consistent with the formation of weak soluble complexes between ETD and CHT [35]. The anionic nature of the drug and the strong positive charge of the polymer at pH < 6.5 [24] favored an electrostatic interactions in addition stabilizing complexation may be assumed. The negative curvature of type AN diagram was probably due to self association phenomena of CHT molecules at higher concentration [35]. The slope of the initial straight line part of the curve, demonstrated the relative affinity of the drug for the polymer [36]. Approximate 15 fold increase in drug solubility was observed in the presence of 2% w/v chitosan. The results of phase solubility study were found in accordance with the established formation of soluble complex between CHT and poorly soluble drugs [25].
Drug content of the prepared binary systems
The drug content of the prepared binary systems were found to be in the range of 99.2 ± 0.35 to 101.1 ± 0.29 % indicating that the present methods for the preparationof solid systems can be applied with high content uniformity.
Micromeritic properties
It is known that in glass, solid solutions and amorphous dispersions, the particle size is reduced to a minimum level. After carrier dissolution, the drug is molecularly dispersed in the dissolution medium resulting in an enhanced dissolution rate [37]. The micromeritic behaviors (mean particle size, span) of pure etodolac and different ETD-CHT binary mixtures are shown in Table I. The data of the table revealed that ETD has larger mean particle size than all other etodolac simples obtained via grinding or kneading technique. The smallest mean particle size was obtained for the kneaded binary mixture containing 5 % w/w of the drug. It is obvious that as the ratio of CHT decreased from 95% w/w to 80% w/w, the particle size of the drug increased from 81.50 to 150 µm in the kneaded mixtures. The results confirm that kneading method is more effective than the co-grinding method in amorphization of ETD. The smaller span values indicating narrower particle size distribution. No significant difference between the different kneading mixtures (P 0.05), while physical mixture differs significantly from co grinding and kneading mixture (P 0.05).
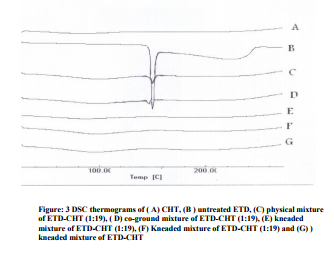
Scanning electron microscopy
The SEM images for pure ETD, 1:19 ETDCHT PM, CG and KM are shown in Figure 2 A-E, respectively. Pure drug image showed prismatic-like structure crystals. The crystalline ETD form was confirmed bySEM, and the CHT presented a regular shape. The PM showed characteristics related to ETD and CHT, On the other hand, particles obtained by different methods as grinding or kneading presented particular morphologies, with reduced sizes when compared to raw materials. The micrograph of ETD-CHT KM did not show the original prismatic crystalline structure of ETD, but showed very small particles thus indicating the amorphization of the drug.
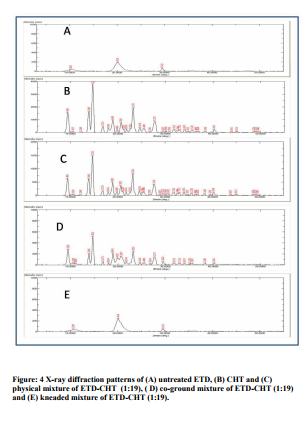
Differential Scanning Calorimetry (DSC) The DSC
thermogram of ETD (Figure 3) exhibited a typical of a crystalline anhydrous substance, showing a sharp endothermic peak (T= 150 °C) corresponding to the melting point of ETD. The DSC trace of CHT was typical an anhydrous amorphous compound. Physical mixture and co grinding mixture revealed the presence of the drug peak with slight shift in the melting temperature of the drug along with significant decrease in the endothermic peak. Kneading mixture showed complete disappearance of the endothermic peak of the drug indicating a change in the nature of ETD from crystalline to amorphous one. The disappearance of the ETD fusion peak observed may be related to a chemical or physical interaction between the drug and CHT or the possible formation of an amorphous system.
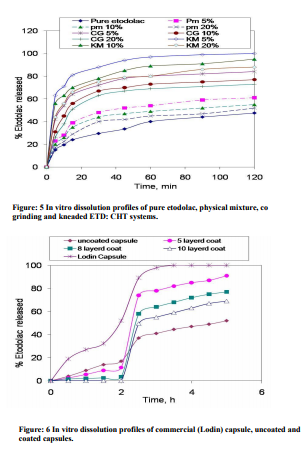
X-ray diffraction studies The XRD analysis
was performed to confirm the results of the DSC study. Diffraction spectra of pure ETD, ETD-CHT physical mixture, co- grinding mixture and kneading he XRPD pattern of the etodolac (Figure 4 B) showed sharp peaks at diffraction angles of 2θ 9.4, 13.9, 14.7, 18.9, 23.2, 27.7, 31.8, 34 and 40.2. The diffractoghrams of the physical mixtures and co grinding mixture were similar to those obtained for pure drug, but the peak size was reduced (Figure 4Cand 4D). Whereas, ETD-CHT kneading mixture (Figure. 4E) did not show these peaks indicating a transition of ETD from a crystalline to an amorphous state. These results, taken together with DSC data, indicate a formation of amorphous ETD:CHT system.mixture are shown in Figure 4. In vitro release studies The effects of varying drug/chitosan ratio and preparation methods of drug: polymer mixture on etodolac dissolution rate is shown in Figure 5. In general, drug dissolution progressively improved with increasing the polymer proportion in the mixture and reached the highest values at the 1:19 w/w drug: polymer ratio. The slight increase in drug dissolution shown by simple physical mixtures could be due to a reduction of the interfacial tension between the hydrophobic drug particles and the dissolution medium, owing to the presence of the hydrophilic polymer, as well as to a local solubilizing effect acting during the early stages of the dissolution process in the microenvironment surrounding the drug particles [36]. The high rate of drug dissolution shown by kneaded mixtures and coground mixtures could be attributed to the intimate physical content between ETD and hydrophilic carrier, to particle size reduction brought about by the mechanical treatment and to a decrease in drug crystallinity during cogrinding with the amorphous carrier [38- 41]. These finding were in agreement with the results of solid state studies, confirming that the best dissolution performance of kneaded products is mainly ascribable to the almost complete drug amorphization achieved in these systems. At this stage of the study, it can be concluded that chitosan can favorably enhance the etodolac dissolution property. Generally, dissolution rate of ETD increased significantly with increasing the polymer concentration in the order of KM> CG> PM> pure drug. Table II illustrates the percent drug dissolved at 30 min, dissolution efficiency at 120 min and relative dissolution rate at 60 min of pure ETD, PM, CG and KM. The data demonstrated that CG and KM exhibited a higher relative dissolution rate at 60 min than PM, when compared to the pure drug. The analysis of release data (Table II) confirms that the dissolution efficiency (DE) was statistically different (P <0.05) for the ETD and PM in comparison with CG and KM complexes, although the different KMs did not show significant results. These results were confirmed by the analysis of t 50% (time in which 50% of ETD is dissolved); the values calculated were basically identical. DE120 permits the better comparison between the dissolution behavior of different binary systems. DE improved to up of about 2.5 times in 1:19 w/w drug:chitosan KM. The amorphizing effect of CHT appeared as the main driving force for the enhanced drug dissolution, even though other factors such improved wettability, reduced aggregation phenomena, increased effective surface area and total solubilization effect played a contribution role. The most effective method of preparation was the kneading technique which showed the best dissolution characteristic. These results are in good agreement with the results obtained by Mishra and Kumar [27], where the mixture of valdecoxib and chitosan prepared by kneading method showed the highest dissolution rate. Whereas, the obtained results were no consistent with that obtained by Mura et al. [42], they concluded that naproxen: chitosan solid systems prepared by coground method demonstrated the best drug dissolution characteristics. The second aim of this study was to prepare convenient colon drug delivery system of ETD. Fast dissolving tablets of the 5% w/w KM was considered unsuited to yield a tablet of appropriate dimensions. For this reason, capsules containing the calculated amount of the mixture equivalent to 100 mg of etodolac were prepared and coated with guar gum solution. Furthermore, the capsule formulation permitted the comparison to the commercial capsule dosage form. Guar molecule consists of a mannose backbone with random galactose substitutions in the ratio of 1.6:1. The galactose units solubilize the polymer through steric effects; galactose poor regions, on the contrary, are less soluble and can associate both intra-and intermolecularly to form partially crystalline complexes. Because of this association, guar possesses remarkable rheological properties [43]. Guar gum in water behaves essentially as a viscoelastic fluid upon addition of isopropyl alcohol its viscoelastic behavior and structural properties are changed dramatically. Isopropyl alcohol promotes the formation of a net-work of large-scale structures via intermolecular association thus increasing dramatically the elastic response of guar gum [43]. Therefore, the step of plasticization was eliminated in addition experimental trials revealed that the coating film was spread smoothly without cracking upon drying. Dissolution behavior of the capsules is presented in Figure 6. At the end of dissolution run in pH 1.2 the uncoated capsules released about 17% w/w of the drug, while coated capsules with coat thickness of 5 and 8 layers reduced the percentage drug release to 11.51 and 3.5 % w/w, respectively. Increasing the coat thickness to 10 layers resulted in complete inhibition of drug release in the acid medium. As the dissolution medium is rendered alkaline to pH of 6.8, significant drug release was observed. Guar gum dissolves in pH 6.8, therefore, the rate of dissolution of the coat and the release of the drug depended on the coat thickness. In general, the coated capsules released their drug content faster than the uncoated capsules in pH 6.8. It can be seen that the drug released from the uncoated capsule after one hour dissolution in phosphate buffer pH 6.8 was 41 % w/w whereas, from the 10 layered coated capsules was 55% w/w. This observation can be explained by the increased dissolution rate of the drug from its chitosan binary system as discussed before. The rates of drug release from the different capsules increased as the coat thickness decreased. It was also observed that after the initial faster drug release from the coated capsules, the drug was assumed to be released in almost a constant rate.
Conclusion
This study showed that chitosan can favorably affect the etodolac dissolution properties, yielding a dissolution efficiency improvement of up to 2.5 and 2.1 times in 1:19 (w/w) ETD: CHT kneaded product and coground mixture, respectively. The amorphizing effect of chitosan appeared as the main driving force for the enhanced drug dissolution, even though other factors such as improved wettability, reduced aggregation phenomena, increased effective surface area and local solubilization effect played a contribution role. The film coating property of guar gum has been investigated to assess its potential in the design of gastroresistant delivery dosage form. This study showed the usefulness of guar gum aqueous coating solution in preventing the release of etodolac in the acidic pH and permitting its release in the colon environment.

References:
. Yang L, Chu JS, Fix JA. Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int J Pharm 2002; 235: 1-15
. 2. Halsas M, Penttinen T, Veski P, Jurjenson H, Marvola M. Time-controlled release pseudoephedrine tablets: bioavailability and in vitro/in vivo correlation. Pharmazie. 2001; 56: 718- 723.
3. Haslas M, Hietala J, Veski P, Jurjenson H, Marvola M. Morning versus evening dosing of ibuprofen using conventional and time-controlled release formulations. Int J Pharm 1999; 189: 179-185.
4. Jemal A, Murray, T.; Ward, E.; et al. Cancer statistics. CA Cancer J .Clin. 2005, 55(1), 10-30.
5. Janne P, Mayer R. Chemoprevention of colorectal cancer. N Engl J Med 2000; 342: 1960-1968.
6. Gupta RA, DuBois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Review 2001; 1: 11-21.
7. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase-2 gene expression in human and colorectal adenomas and adenocarcinomas. Gastroenterology 1994; 107: 1183-1188.
8. Fujita T, Matsui M, Takaku K. Size-and invasion-dependent increase in cyclooxygenase-2 levels in human colorectal carcinomas. Cancer Res 1998; 58: 4823-4826.
9. El-Kamel AH, Abdel-Aziz AA, Fatani AJ, El- Subbagh HI. Oral colon targeted delivery systems for treatment of inflammatory bowel diseases: synthesis, in vitro and in vivo assessment. Int J Pharm 2008; 358(1-2): 248-255.
10. Asghar, LF, Chandran S. Design and evaluation of pH modulated controlled release matrix systems for colon specific delivery of indomethacin. Pharmazie 2008; 63(10): 736-742.
11. Krishnaiah YS, Satyanarayana V, Kumar BD, Karthikeyan RS. Studies on the development of colon-targeted delivery systems for celecoxib in the prevention of colorectal cancer. Drug Target. 2002; 10(3): 247-254.
12. Takeda J, Kitajima K, Fujii S, Horiuchi H, Hori H, Chibana Y, Okuyama T, Tominaga K, Ichikawa K, Ono Y, Teramoto T, Ohkura Y, Imura J, Shinoda M, Chiba T, Sakamoto C, Kawamata H, Fujimori T. Inhibitory effects of etodolac, a selective COX-2 inhibitor, on the occurrence of tumors in colitis-induced tumorigenesis model in rats. Oncol Rep 2004; 11(5): 981- 985.
13. Use of etodolac for the treatment of prostate cancer. United States Patent 710556.
14. Mishima K, Nariari Y, Yoshimura Y. Etodolac, a selective cyclo-oxygenase-2 inhibitor, enhances carboplatin-induced apoptosis of human tongue carcinoma cells by down-regulation of FAP-1 expression. Oral Oncology 2005; 4: 77-81.
15. Yanaoka K, Oka M, Yoshimura N, Deguchi H, Mukoubayashi C, Enomoto S, Maekita T, Inoue I, Ueda K, Utsunomiya H, Iguchi M, Tamai H, Fujita shiro, Nakamura Y, Tsukamoto T, Inada K, Takeshita T, Ichinose M. Preventive effects of etodolac, a selective cyclooxygenase-2 inhibitor, on cancer development in extensive metaplastic gastritis, a Helicobacter pylori-negative precancerous lesion. Int J Cancer 2010; 126(6): 1467-1473.
16. Okamoto A, Shirakawa T, Bito T, Shigemura K, Hamada K, Gotoh A, Fujisawa M, Kawabata M. Etodolac, a selective cyclooxygenase-2 inhibitor, induces upregulation of E-Cadherin and has antitumor effect on human bladder cancer cells in vitro and in vivo. Urology 2008; 71(1): 156-60.
17. Martindale. The Complete Drug Reference, 32th Edition, 1999.
18. Lobenberg R, Amidon GL. Modern bioavailability, Bioequivalence and Biopharmaceutics Classification System. New Scientific Approaches to International Regulatory Standards. Eur J Pharm Biopharm 2000; 50: 3-12.
19. Ozkan Y, Doganay N, Diknen K, Isimer A. Enhanced release of solid dispersion of etodolac in polyethylene glycol. Farmaco 2000; 55: 433-438.
20. Brunella C, Clelia di M, Maria L, Agnese M, Antonio C. Etodolac/cyclodextrin formulation:physicochemical characterization and in vivo pharmacological studies. Drug Dev Ind Pharm 2009; 35: 877-886.
21. Sinha VR, Amita RC, Honey G. Enhancing the dissolution of hydrophobic guests using solid state inclusion complexation: characterization and in vitro evaluation. J Inclusion Phenomena and Macrocyclic Chemistry 2010; 66: numbers 3-4 April.
22. Barakat NS. Enhanced oral bioavailability of Etodolac by selfemulsifying system: in vitro and in vivo evaluation. J Pharm Pharmacol 2010; 62: 173-180.
23. Paul W, Sharma CP. Chitosan, a drug carrier for the 21st century. STP Pharma Sci 2000; 10: 5-22.
24. Portero A, Remunan-Lopez C, VilaJato JL. Effect of chitosan and chitosan glutamate enhancing the dissolution properties of the poorly water soluble drug nefedipine. Int J Pharm1998; 175: 75-84.
25. Mishra DN, Kumar SGV, Zerrouk N, Mennini N, Maestrellis F, Chemtob C. Development and characterization of naproxen-chitosan solid systems with improved drug dissolution properties. Eur J Pharm Sci 2003; 19: 67-75.
26. Mutalik S, Anju P, Manoj K, Usha AN. Enhancement of dissolution rate and bioavailability of aceclofenac: A chitosan-based solvent change approach. Int J .Pharm 2008; 350: 279- 290.
27. Mishra DN, Kumar SGV. Preparation, Characterization and in vitro dissolution studies of solid systems of valdecoxib with chitosan. Chem Pharm Bull 2006; 54: 1102-1106.
28. Fukuda M, Peppas NA, McGinity JW. Properties of sustained release hot-melt extruded tablets containing chitosan and xanthan gum. Int J Pharm 2006; 310: 90-100.
29. Rama Prasad YV, Krishnaiah YSR, Satyanarayana S. In vitro evaluation of guar gum as a carrier for colon-specific drug delivery. J Control Release 1998; 51: 281-287.
30. Krishnaiah YSR, Satyanarayana S, Rama Prasad YV, Narasimha Reo S. Evaluation of guar gum as a compression coat for drug targeting to colon. Int J Pharm 1998; 171: 137- 146.
31. Krishnaiah YSR, Bhaskar Reddy PR, Satyanarayana V, Karthikeyan RS. Studies on the development of oral colon targeted drug delivery systems for metronidazole in the treatment of amoebiasis. Int J Pharm 2002; 236: 43- 55.
32. Krishnaiah YS, Satyanarayana V, Dinesh Kumar B, Karthikeyan RS. In vitro drug release studies on guar gumbased colon targeted oral drug delivery systems of 5-fluorouracil. Eur J Pharm Sci 2002; 16(3):185-192.
33. Rane S, Kale V. Evaluation of modified guar gum as film coating material. Int J Chem Tech Research 2009; 1: 180-182.
34. Tobiska S, Kleinbudde P. Coating Uniformity: Influence of Atomizing Air Pressure. Pharm Dev Technol 2003; 8: 39-46.
35. Higuchi T, Connors KA. Phase solubility techniques. Adv Ana Chem Instr 1965; 4: 117-210.
36. Najib NM, Suleiman MS. Characterization of a diflunisal polyethylene glycol solid dispersion system. Int J Pharm 1989; 51: 225-232.
37. Adamson, A. W. Physical chemistry of surfaces, third ed., Wiley, New York. 1976.
38. Sawayanagi Y, Nambu N, Nagai T. Dissolution properties and bioavailability of griseofulvin from ground mixtures with chitin or chitosan. Chem Pharm Bull 1982; 30: 4454-4467.
39. Sawayanagi Y, Nambu N, Nagai T. Dissolution properties and bioavailability of phenytoin from ground mixtures with chitin or chitosan. Chem Pharm Bull 1983; 31: 2062-2068.
40. Acerturk F, Sencan A, Celebi N. Enhancement of the dissolution of spiranolactone with chitosan and low molecular weight gelatin. STP Pharma Sci. 1993; 3: 369-373.
41. Acerturk F, Sencan A, Celebi N. Evaluation of the effect of low molecular weight chitosan on the solubility and dissolution characteristics of spiranolactone. Pharmazie 1993; 48: 605-607.
42. Mura P, Zerrouk N, Mennini N, Maestrellis F, Chemtob C. Development and characterization of naproxen-chitosan solid systems with improved drug dissolution properties. Eur J Pharm Sci 2003; 19: 67-75.
43. Gittings MR, Cipelletti L, Trappe V, Weitz DA. The effect of solvent and ions on the structure and rheological properties of Guar solutions. J Phys Chem A 2001; 105: 9310-9315.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License