IJCRR - 2(10), October, 2010
Pages: 27-33
Print Article
Download XML Download PDF
SPECTROPHOTOMETRIC DETERMINATION OF SPARFLOXACIN IN BULK AND DOSAGE FORMS
Author: Okorie HN, Mbah CJ
Category: Healthcare
Abstract:A spectrophotometric study of sparfloxacin is described. The method is based on the charge-transfer complexation between sparfloxacin as n-electron donor with chloranilic acid as -acceptor to form a violet-coloured complex having absorption maximum at 530 nm. Beer?s plot is obeyed in the concentration range of 5-30 g/ml. Results of theanalysis of this method were validated statistically by recovery studies. The proposed
method is simple, accurate and precise for the quantitative determination of sparfloxacin in bulk and tablet formulations.
Keywords: Sparfloxacin, spectrophotometry, chloranilic acid.
Full Text:
INTRODUCTION
Sparfloxacin, 5-amino-1-cyclopropyl-7- (cis-3, 5-dimethyl-1-piperazinyl)-6,8- difluoro-1, 4-dihydro-4-oxo-3- quinolinecarboxylic acid is a difluoroquinolone antibacterial agent belonging to the third generation quinolones. Clinically, it is very effective in the treatment of streptococci infections. Its mechanism of action involves the inhibition of DNA synthesis by promoting cleavage of bacterial DNA in the DNA-enzyme complexes of DNA gyrase and type iv topoisomerase, resulting in rapid bacterial death1 . The drug is not official in any pharmacopoeia, hence no official method is available for the estimation of the drug in formulations. A number of analytical methods used for the determination of sparfloxacin in pure and dosage forms include electrochemistry2-5 ; UV-Visible spectrophotometry6-9 ; HPLC 10-13 . Chloranilic acid, 2,5-dichloro-3,6- dihydroxy-p-benzoquinone has been used largely as a spectrophotometric reagent for the determination of some organic compounds containing lone pair of electrons14-18, but not hitherto in the assay of sparfloxacin. The present work describes the spectrophotometric determination of sparfloxacin in bulk and pharmaceutical formulations using chloranilic acid as a chromogenic reagent.
MATERIALS AND METHODS
Materials: Sparfloxacin (Micro Labs Limited.92, Sipcot, Hosur. India), all other chemicals were of analytical grade. Freshly prepared 0.5 % (w/v) chloranilic acid solution in dioxan. A Hitachi UV/VIS spectrophotometer, model 2000 (Japan) was used for absorbance measurements.
Standard solution: A stock solution of sparfloxacin (50 g/ml) was prepared by dissolving the required amount in methanol. Standard solutions of the analyte (5-30 g/ml) were prepared by serial dilution of the stock solution.
Proposed procedure: An aliquot of the standard solution containing sparfloxacin was transferred into a 10 ml volumetric flask. A 1-ml volume of chloranilic acid solution (500 g/ ml) was added and the contents were mixed thoroughly. After 30 min standing, the volume was made up with dioxan and the absorbance of the solution was measured at 530 nm against reagent blank.
Procedure for assay of dosage forms: Ten tablets of the drug were weighed and ground to a fine powder. An adequate amount of the powder was transferred into a beaker. The powder was dissolved in methanol by stirring for 15 min. The mixture was filtered to a volumetric flask (100 ml) through Whatman filter paper No.41. The filtrate and washings were diluted to volume with methanol. A suitable volume of this solution was treated as described under proposed procedure and the drug content was evaluated. The results are given in Table 2
Procedure for recovery of sparfloxacin: To study the recovery of sparfloxacin, samples were prepared by mixing known amounts of pure sparfloxacin with portions of commercial preparation. The mixtures obtained were assayed by proposed method and the results are presented in Table 3.
RESULTS AND DISCUSSION Spectrophotometric characteristic of the sparfloxacin-chloranilic acid system: A violet-coloured complex with a ratio of sparfloxacin to reagent of 1:1 was formed when chloranilic acid solution was added to sparfloxacin solution. The complex exhibited a max at 530 nm while the reagent showed a max at 434 nm. An absorbance of the complex using an aliquot of the standard solution was measured at 530 nm at 30 min interval over a period of 2h. No change in the initial absorbance was observed indicating that the colour of the complex is fairly stable. Beer?s law was obeyed in the range of 5-30 g/ml. Beer?s law range, molar absorptivity, slope, linear least-square analysis are given in Table 1.
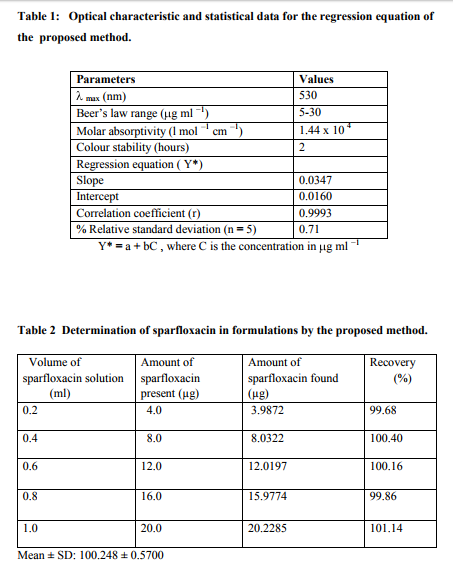
Optimization of reaction conditions: Effect of chloranilic acid concentration: The effect of chloranilic acid concentration on the colour development was studied. It was observed that 1 ml of 0.5 % (w/v) chloranilic acid solution produced maximum colour intensity (Fig. 1).
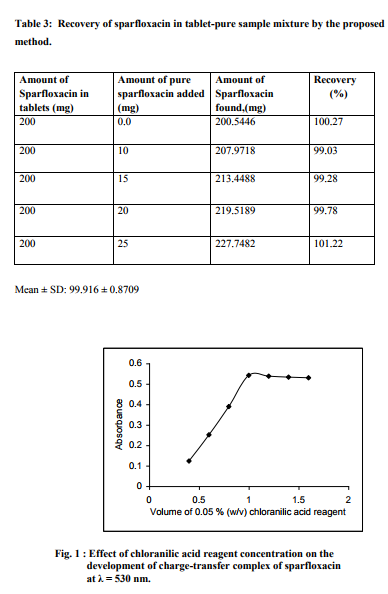
Effect of reacting time: The colour product developed rapidly after addition of the reagent attaining maximum intensity after 30 min at room temperature (Fig. 2). The colour was stable for over 2 h.
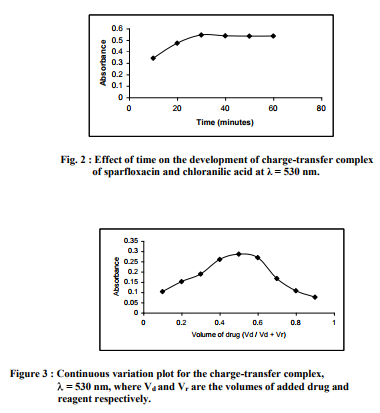
Stoichiometric relationship: In order to establish the composition of the charge-transfer complex, the molar ratio method and Job?s method of continuous variation using equimolar solutions of the drug (0.004 M) and reagent (0.004 M) were studied. In the molar ratio method, the concentration of the drug was kept constant while varying the concentration of the reagent in the series of solutions prepared. In the Job?s method, equimolar solutions of the drug and the reagent were mixed in complimentary proportions to a fixed total volume. The results obtained indicate that the composition of chargetransfer complex was (1:1) drug to reagent (Fig. 3). In conclusion, the proposed spectrophotometric method was applied in the determination of sparfloxacin in bulk and pharmaceutical formulations. The method is simple, accurate, reproducible and the statistical analysis has good agreement with reported methods. The optimum conditions for the proposed method have been established and the method has shown a reasonable tolerance towards excipients. Finally, due to the minimum time required for the complexation to be complete, the proposed method can be employed for the routine analysis of sparfloxacin from bulk and tablet dosage form in quality control laboratories.
References:
1. Hooper DC. Mode of action of fluoroquinolones. Drugs 1999;58(2): 6-10.
2. Abdel-Ghana NT, EL-Shall MA, El-s MA. Validated polarographic method for the determination of certain antibacterial drugs. Anal Sci 2007;23:1053-57.
3. Kumar KG, Augustine P, Poduval R, John S. Voltammetric studies of sparfloxacin and application to its determination in pharmaceuticals. Pharmazie 2006;61:291-92.
4. Jains NK, Pitre KS. Electrochemical analysis of sparfloxacin in pharmaceutical formulations and biochemical screening of Co (II) complex. J Pharm Biomed Anal 2002;29:795-801.
5. Reedy TM, Sreedhar M, Reedy J. Electrochemical determination of sparfloxacin in pharmaceutical formulations and urine samples using -cyclodextrin modified carbon electrode. Anal Lett 2003;36:1365-79.
6. Akram M, EL-Didamony AM. Spectrophotometric determination of sparfloxacin in pharmaceutical preparations by ternary complex formulation with Pd (II) and eosin. Anal Lett 2007;40:2708-20.
7. Kuchekar BS, Thakkar SV, Chothe PP, Herimathm MR, Shinda DB. Spectrophotometric estimation of sparfloxacin in tablets. Indian J Pharm Sci 2002;64: 496-7.
8. Herida RN, Marona HRN, Elfrides ES, Schapoval EES. Spectrophotometric determination of sparfloxacin in pharmaceutical formulations using bromothymol blue. J Pharm Biomed Anal 2001;26:501-4.
9. Marona HRN, Schapoval EES. Spectrophotometric determination of sparfloxacin in tablets. J Antimicob Chemother 1999;44:136-7.
10. Nahar NR, Shahabuddin A. Development of quantitative analysis of sparfloxacin by high performance liquid chromatography. J Pharm Pharmaceut Sci 2007;6:21-7.
11. Marona HRN, Schapoval EES. A high performance liquid chromatographic assay of sparfloxacin. J Pharm Biomed Anal 1999;20:413-7.
12. El-Sayed YM. A simple high performance liquid chromatographic assay for sparfloxacin in human plasma. Anal Lett 1995;28:279-93.
13. Borner KE, Lode H. Determination of sparfloxacin in serum and urine by high performance liquid chromatography. J Chromatogr Biomed Appl 1992;579:285-9.
14. Walash MEM, Sharaf EM, Meturalli M, Redashabara M. Spectrophotometric determination of nizatidine and ranitidine through charge-transfer complex formation. J Arch Pharm Res 2007;27:720-6.
15. Attama AA, Nnamani PO, Adikwu MU, Akidi FO. Spetrophotometric determination of haloperidol by charge-transfer interaction with chloranilic acid. STP Pharm Sci 2003;13:419-21.
16. Adikwu MU, Oforkansi KC. Spectrophotometric determination of moclobemide by charge-transfer complexation. J Pharm Biomed Anal 1997;16:527-32.
17. El-Sayed MA, Barary M, AbdelSalam MA, Mohamed YA. Spectrophotometric assay of cardiovascular drugs through charge-transfer. Ana Lett 1984;22:1651-84.
18. El-Sayed MA, Agarwal SP. Spectrophotometric determination of atropine, pilocarpine and strychnine with chloranilic acid. Talanta 1982;29:535-7.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License