IJCRR - 2(12), December, 2010
Pages: 11-24
Print Article
Download XML Download PDF
PHARMACOGNOSTIC AND PHYTOCHEMICAL INVESTIGATION OF CHLOROPHYTUM BREVISCAPUM
DALZ.
Author: V. N. Patil, Deokule S. S.
Category: General Sciences
Abstract:Chlorophytum breviscapum Dalz. belongs to family Liliaceae and is being used in the indigenous systems of medicine as a galactogogue and aphrodisiac. The drug part is usually used as the white tuberous roots of this plant. The present study includes the macro and microscopic characters, histochemistry and phytochemistry. The phytochemical screening is also confirmed by HPTLC analysis for saponins and stegmasteroids.
Keywords: Chlorophytum breviscapum, Pharmacognosy, phytochemical analysis, HPTLC.
Full Text:
INTRODUCTION
Chlorophytum breviscapum Dalz. belongs to family Liliaceae. In India, it is found to be growing in rain fed areas. The plant generally grows along the forest margins, grassy slopes and rocky places along valleys (between 1300- 2800 m)1 . The plant body is erect up to 1.5- 2 ft height with sheathing leaf base acute to acuminate with entire margin. Tuberous root are slightly broad at the base and gradually tapering at the end. Tubers are oblong, pendulous in the middle again it becomes fibrous and are measuring 8-12 cm long, 1-1.7 cm diameter 2 . There are 17 species of Chlorophytum recorded in India out of these 11 species of Chlorophytum are found to be growing in Maharashtra (3) . For the present investigation Chlorophytum breviscapum is selected as for their correct botanical identification and standardization of drug. The drug part is usually used as the white tuberous roots. The drug is used an aphrodisiac and galactogogue4,5,6 . Review of literature revealed that it is also used as a nutritive and health promoting properties as well as an immunoenhancing, hepatoprotective and antioxidants 7,8,9,10,11. The tubers are also used as a medicinal expectorant in fever, leucorrhoea and also as an aphrodisiac12.
MATERIAL AND METHODS
Collection and Identification of Plant Materials
The plant materials were collected from in and around Pune district of Maharashtra during rainy season. Efforts were made to collect the plants in flowering and fruiting condition for the correct botanical identification. It was identified with help of Flora of The Presidency of Bombay2. The herbariums were prepared and finally authenticated from Botanical Survey of India, Western Circle, Pune (India). The voucher specimen number is PAVICH3/ 2009.
Microscopic and Macroscopic evaluation
Thin (25p) hand cut sections were taken from the fresh tuberous roots, permanent double stained and finally mounted in Canada balsam as per the plant microtechniques method of Johansen13. The macroscopic evaluation was studied by the following method of Trease and Evans14 and Wallis15.
Histochemical study
The thin transverse sections of fresh root were taken (about 25p). It was treated with respective reagent for the detection and localization of chemicals in the tissues as per the method of Krishnamurthy16.
Phytochemical evaluation:
Some materials were dried under the shade so as to avoid the decomposition of chemical constituents, powdered in blender and finally stored in dry air tied containers for phytochemical screening. Ash and percentage extractives were accomplished by following standard pharmacopoeal techniques17. Fluorescence analysis was carried out as per Chase and Pratt18. Qualitative phytochemical test were carried out by standard methods of Harborne19 and Trease and Evans14. Quantitative phytochemical analysis were determined for proteins, carbohydrates and saponins by the methods of Lowry et al., 20; Nelson21 and Obadoni and Ochuko22 respectively. The phytochemical screening was also detected by the High PerformanceThin Layer Chromatography (HPTLC). HPTLC study was carried out on instrument comprising of Linomat 5 for application using Densitometer- TLC Scanner 3 with "WINCATS" software (Camag, Switzerland). These studies were carried out on pre-coated aluminum fluorescent plates (E. Merck). For HPTLC studies, an extract of methanol (25% GR) solvent system was used and after development, plate was scanned at 254 and 366 nm 23, 24, 25.
RESULTS AND DISCUSSIONS
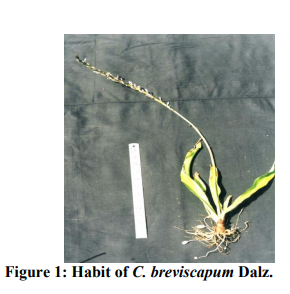
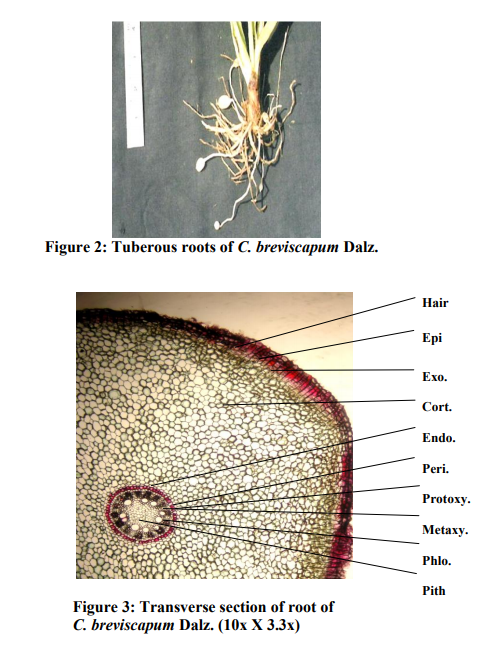
Macroscopic evaluation (Figure 1 and 2)
Herb: 1.5 - 2 ft. in height.
Roots: Tuberous root are slightly broad at the base and gradually tapering at the end. Tubers are oblong, pendulous in the middle again it becomes fibrous and are measuring 8-12 cm long, 1-1.7 cm diameter.
Leaves: Green, 6 - 9 (10-15 also) in number, slightly thick dark green, flat with undulate margin, apex acuminate, linear, oblong or lanceolate,membranous, shining above with sheathing leaf base. 60 - 66 2.70 - 3.5 cm long.
Scape: Unbranched, Naked, 60 - 65 cm long. Flower: White, racemose, clusters of 2 - 3 flowers, 2 - 4 cm long, erect.
Bract: Membranous, Ovate- lanceolate, lower bracts 0.5 - 1.5 cm and upper 0.8 cm long. Pedicels: 0.5 - 0.8 cm long, jointed near the top.
Perianth: Segments, linear acute, 3 nerved, 0.9 0.3 cm in broad. Stamen: 0.5 - 1 cm long, anther- 0.7 cm long, linear, oblong.
Style: 0.6 cm long, slender, stigma minute. Capsule: Globose, 0.8 - 1.2 cm in diameter, 3 winged, emarginate, (0.7 - 0.8 cm in height and 0.8 cm in diameter)
Seeds: Black, globose, compressed 0.1 - 0.3 cm diameter, papillose.
Microscopic characters
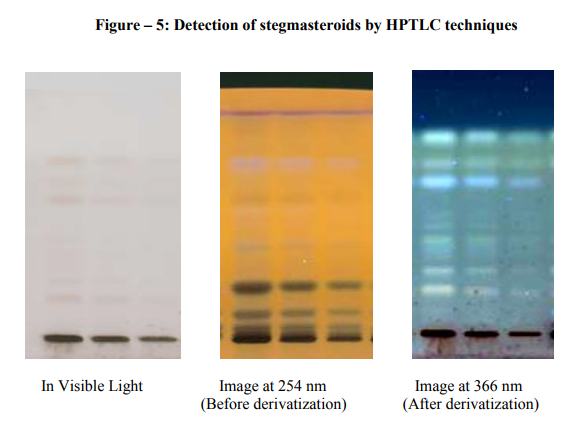
The transverse section of root shows a circular in outline. The outermost layer is epidermis. Epidermis consists of uniseriate trichomes. This is followed by a very large zone of cortex. The outermost layer of the cortex just below the epidermis consists of cells which are mostly rectangular, appearing much longer than wide. The rest of the cortical cells are rounded to polygonal parenchymatous and have no intercellular spaces. The innermost layer of the cortex is single layered endodermis. The stellar structure shows that the endodermis is followed by the pericycle layer. Xylem is exarch type. The phloem is group in between the xylem along with the parenchyma. The centre region is occupied by large pith. These are mostly polygonal in shape (Figure 3).
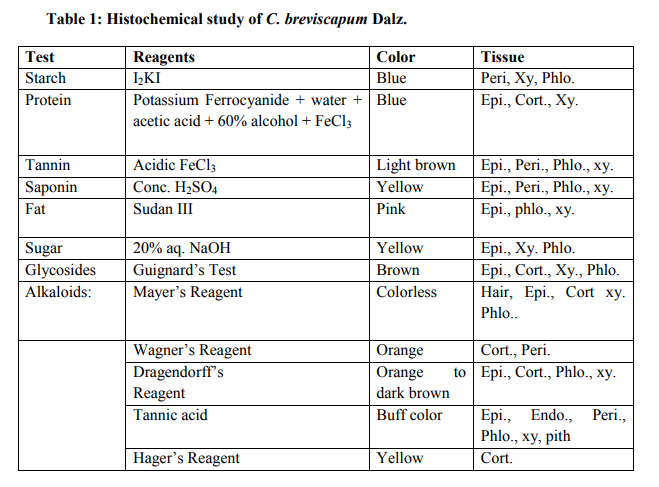
Histochemical Screening
Histochemical screening showed the presence of starch, protein, fat, saponins, tannin, sugars and alkaloids (Table 1).
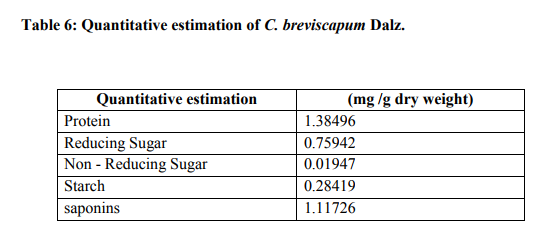
Phytochemical Study
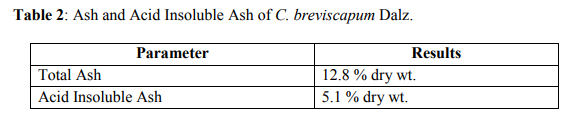
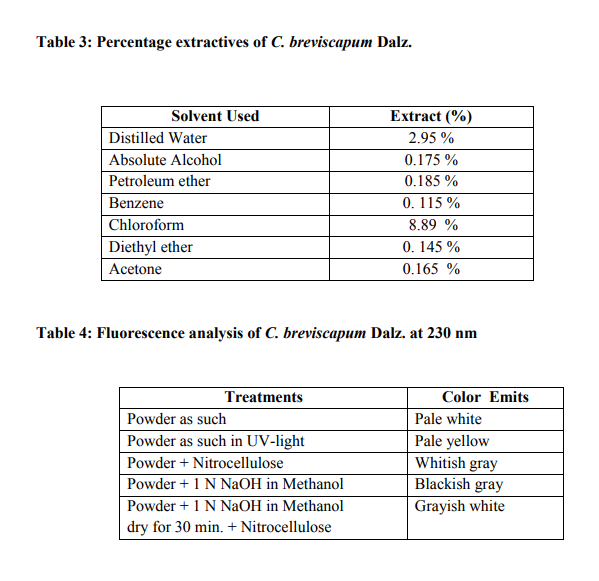
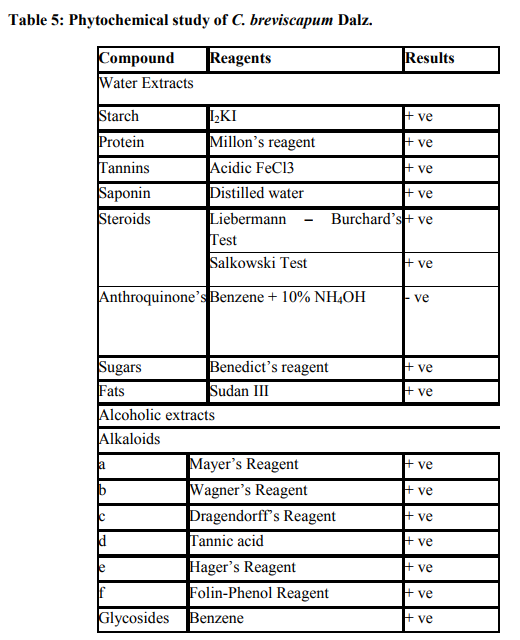
It contains the total ash 12.8 % and acid insoluble ash is 5.1 % (Table 2). The values of percentage extractives were higher in chloroform and lower in benzene solvent (Table 3). Fluorescence analysis was carried out to check the purity of the drug. The powder drug was observed in visible light as Pale white in color. The powder was then observed in ultraviolet light. It was treated with reagent like nitrocellulose, 1 N sodium hydroxide, 1 N sodium hydroxide in nitrocellulose and dry for 30 minutes and then it was observed under ultraviolet light and it emits the color as shown in (Table 4). Qualitative analysis of the root drug indicated the presence of proteins, reducing and non-reducing sugars, saponins, fats, tannin, glycoside and alkaloids in the plant (Table 5). The quantity of proteins is higher than saponins and carbohydrates (Table 6). Saponins are the important chemical and justify the use of tubers of this plant and are used as a well known health tonic, aphrodisiac and galactogogue3,4,6,26. In HPTLC study the methanolic extract is ultrasonic for 15 minutes and filtered. The filtrate is used as an application for saponins and stegmasteroids. For each application 20 p, 10p and 5p extracts were used and loaded on instrument comprising of Linomat 5 for application using Densitometer- TLC Scanner 3 with "WINCATS" software (Camag, Switzerland). These studies were carried out on pre-coated aluminum fluorescent plates (E. Merck). The plates were scanned at 254 and at 366 nm23, 24.
Analytical studies (Saponins)
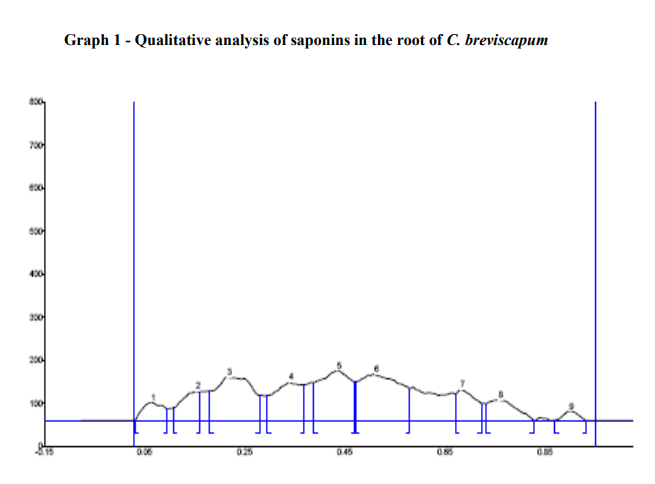
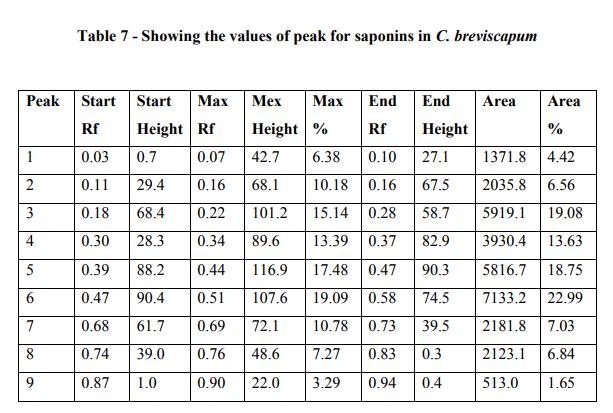
The HPTLC analysis showed that, the saponins from the C. breviscapum samples gave light yellow bands in visible light and blue bands after derivatization in fluorescence light. The plates were scanned at 254 and 366 nm. When images were compared with the graph and table values. It showes maximum area 22.99 % at 366nm after derivatization. The table also indicates the starting Rf values and end Rf values (Figure 4; Graph 1; Table 7).
Analytical studies (Stegmasteroids)
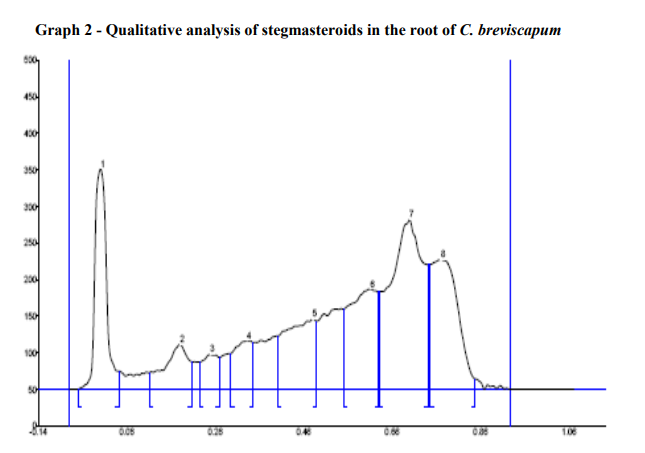
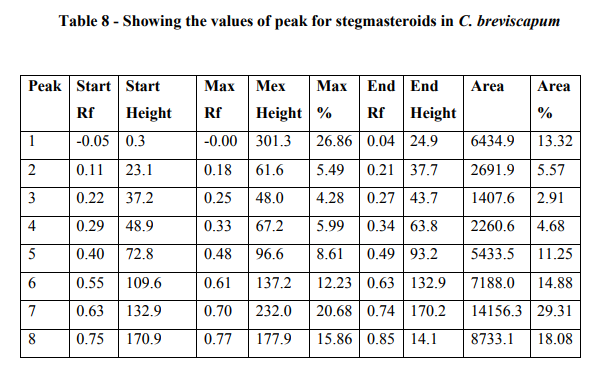
In HPTLC analysis, stegmasteroids revealed white bands in visible light. After derivatization in fluorescence light it showed the dark blue bands. The plates were scanned at 254 and 366 nm. It was covered the area 29.31% at 366nm after derivatization. The tables also indicate the Rf values for all the peaks scanned by "WINCATS" software (Figure 5; Graph 2; Table 8).
CONCLUSION
The plant C. breviscapum showed the correct taxonomy. The morphological characters and histochemical study with double staining of the root, percentage extractives, fluorescence and ash analysis and the phytochemical screening of the plant is helpful for the standardization of drug. As in case of saponins and stegmasteroids, the peaks are denoted by the Rf values. Finding the over all results of the study of C. breviscapum, these investigations will be useful for the correct botanical identification and authentication of the drug.
ACKNOWLEDGEMENT
Both the authors would like to express a sincere thank to Head, Department of Botany, University of Pune, Pune-411 007 for encouragement and necessary laboratory facilities and The first author is grateful to authorities of Pune University for providing financial support in the form of research stipend.

References:
1. Hara H. The Flora of Eastern Himalaya. Tokyo University Press, Japan. 1966. p. 407.
2. Cooke T. Flora of Presidency of Bombay. B.S.I. Calcutta. 1958; 3: 280-289.
3. The Wealth of India- A dictionary of Indian raw materials and industrial products,. Revised Edn. Publication and Information Directorate, CSIR. Dr. R.S. Krishnan Marg, New Delhi. 1992.
3 (Ca-Ci): 482-483.
4. Nadkarni AK. K. M. Nadkarni?s Indian Materia Medica. Popular Book Depot., Lamington Road, Bombay, 3rd Edtn. 1927. 1: 208-209.
5. Chopra RN, Nayer SL, Chopra IC. Glossary of Indian medicinal Plants CSIR. New Delhi. 1956. p. 218.
6. Marais W, Reilly J. Chlorophytum and its related Genera (Liliaceae). Kew Bulletin. 1978; 32 (3): 653-663.
7. Govindarajan R, Vijayakumar M, Pushpangadan P. Antioxidant approach to disease management and the role of Rasayana? herbs of Ayurveda. Journal of Ethnopharmacology. 2005; 99: 165- 178.
8. Anonymous. Medicinal Plants more on Safed Musali. Agriculture and Industry Survey. 2001 May. p. 38- 39.
9. Dhuley JN. Effect of some Indian herbs on macrophase functions in Ochratoxin A treated mice. Jour. of Ethnopharmacology. 1997; 58: 15- 20.
10. Nergard CS, Diallo D, Michaelsen TE, Malterud KE, Kiyohara H, Matsumoto T, et al. Isolation, Partial characterization and immunostimulation activity of polysaccharides from Verninia kotschyana Sch. Bip. Ex. Walp. Journal of Ethnopharmacology. 2004; 91: 141-152.
11. Kirtikar KR, Basu BD. Liliaceae: Chlorophytum. In: Kirtikar, K.R.; Basu B.D. (Eds.) Indian Medicinal Plants. L.M. Basu Publishers. Allahabad, India. 1975. 2508-2509.
12. Sreevidya N, Kumar V, Kumar S, Sikarwar RLS. Utilization, depletion and conservation of Safed Musali (Chlorophytum spp.). Journal of non- Timber Forest Products. 2003; 10: 155-157.
13. Johansen DA. Plant Microtechnique, McGraw-Hill Book Co. Inc. New York. 1940. p. 151-154, 182-203.
14. Trease GE, Evans WC. Trease and Evans Pharmacognosy, 15th Ed. W. B. Saunders Edinburgh London, New York, Philadelphia St. Louis Sydney Toronto. 2002. p. 3-4, 528- 533, 538-547.
15. Wallis TE. A Text Book of Pharmacognosy, reprinted edition, Churchill, Livingstone. London. 1967. p. 578 - 617. 16. Krishnamurthy KV. Methods in the Plant Histochemistry, Viswanadhan Pvt. Limited, Madras. 1988. p. 1 - 77.
17. Anonymous. Pharmacopoeia of India, Government of India, Ministry of Health Manager, Publications Delhi, 1st Edn. 1955. p. 370 and 864.
18. Chase CR, Pratt R. Fluorescence of powdered vegetable drugs with particular reference to development of a system of identification. Jour. Of Amer. Phar. Asso. (Sci. ed.). 1949; 38: 324-330.
19. Harborne JB. Phytochemical Methods, Chapman and Hall International Edition, London. 2nd edition. 1973. p. 5-8.
20. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin-Phenol reagent. Jour. of Biol. Chem. 1951; 193: 265-275.
21. Nelson N. A photometric adaptation of the Somogyi method for the determination of Glucose. Jour. of Biol. Chem. 1944; 153: 375-380.
22. Obadoni BO, Ochuko PO. Phytochemical studies and comparative efficacy of crude extracts of some homeostatic plants in Edo and Delta state of Nigeria. Global Jour. Pure Appl. Sci. 2001; 8: 203-208.
23. Wagner H, Baldt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas. Springer - Verlag, Berlin. 1996. p. 129, 144, 155, 157, 176, 178, 206.
24. Reich E, Schibii A. High Performance- Thin Layer Chromatography for the analysis of medicinal plants, Thieme medical publishers. Inc. 2007. p. 129-160, 206-210, 224-240.
25. Patil VN, Deokule SS. Pharmacognostic standardization of Chlorophytum bharuchae Ans. et al. International Journal of Current Research, 2010, 3, 27-32.
26. Oudhia P. Problem perceived by Safed Musali (Chlorophytum borivilianum) growers of Chhattisgarh (India) region: A study. Journal of Medicinal and Aromatic Plant Sciences. 2001; 22/4A and 23/ 1A: 396-399.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License