IJCRR - 2(12), December, 2010
Pages: 03-10
Print Article
Download XML Download PDF
SIMULTANEOUS SPECTROPHOTOMETRIC ESTIMATION OF THIOCOLCHICOSIDE AND DICLOFENAC POTASSIUM B.P. IN COMBINED DOSAGE FORM BY RATIO DERIVATIVE AND DUAL WAVELENGTH METHOD
Author: Choudhari V.P., Chabukswar A.R., Savakhande S.N., Tryambake M.U., Suryawanshi V.M., Sayal P.K.
Category: Healthcare
Abstract:A simple, economical, precise and accurate method for simultaneous determination of Diclofenac potassium (DICLO K) and Thiocholchiside (THIO) in combined dosage form has been developed. The first method is based on Ratio Derivative (Method A)
and second method is based on Dual Wavelength (Method B). The amplitudes 268.78 nm and 355.62 nm wavelength were selected to determine THIO and DICLO K respectively, by method A. In method B, two wavelengths were selected for each drug in a way so that the difference in absorbance is zero for another drug. At wavelengths 252.47 nm and 260.74 nm Thiocholchiside have equal absorbance therefore these two wavelengths were used to determine DICLO K and on similar basis 263.22 nm and
301.65 nm were selected to determine THIO in combined formulation. The drugs obey Beer?s law in the concentration range of 12.5-75 \?g mL-1 for DICLO K and 2-12\?g mL- 1 for THIO by the method A and 25-75 \?g mL-1 for DICLO K and 4-12 \?g mL-1 for
THIO by the method B. The % assay of DICLO K and THIO was found to be in the range 99.95 \? 100.29 % by the proposed methods. Recovery was found in the range of 98.6 - 101.0 % for both analytes by both methods. The results of analysis have been
validated statistically and recovery studies confirmed the accuracy and reproducibility of the proposed methods which were carried out by following ICH guidelines.
Keywords: Diclopfenac potassium (DICLO K) Thiocholchiside. (THIO), Ratio Spectra Derivative Spectrophotometry, Dual wavelength Spectrophotometry.
Full Text:
INTRODUCTION
Diclofenac potassium, potassium [0- (2,6-dichloroanilino) phenyl] acetate, is non-steroidal anti-inflammatory agent. Diclofenac is officially in B.P. where as its potassium salt is official in European pharmacopeia. Other method reported for its estimation are by laser desorption ionisation mass spectra, thermal and fractional analysis, UV Spectrophotometry method, Direct rapid and sensitive HPLC and HPTLC.
Thiocolchicoside is (s)-N-[3-(B-Dglucopyranoxyloxy)-5, 6, 7, 9- tetrahydro-1,2-dimethoxy-10- (methylthio) -9-oxobenzo[a]heptalen- 7yl] acetamide (Fig.1). Thiocolchicoside is a synthetic sulphur derivative of colchicoside. Thiocolchicoside has a selective affinity for γ-amino- butyric acid (GABA) receptors and acts on the muscular contracture by activating the GABAnergic inhibitory pathways thereby acting as a potent muscle relaxant4 . For THIO various analytical methods have been reported or its individual estimation and in combined dosage form which includes HPLC, electrophoresis, LC-MS, extractive Spectrophotometry, visible Spectrophotometry, HPLC with electrochemical detection. Two methods have been reported for simultaneous analysis of DICLO K and THIO in its combination which includes TLC- Densitometry and second order derivative Spectrophotometry. Here an attempt has been made to develop simple, rapid and accurate Dual Wavelength and Ratio Derivative spectroscopic methods for simultaneous estimation of DICLO K and THIO from its formulation. The proposed methods are optimized and validated as per International Conference on Harmonization (ICH) guidelines.
MATERIALS AND METHODS
Instrumentation An UV-Visible double beam spectrophotometer (Varian Cary 100) with 10 mm matched quartz cells was used for Spectrophotometric method. All weighing were done on electronic balance (Model Shimadzu AUW-220D). Spectroscopic grade methanol was used throughout the study. Ultrasonicator (Model 5.5 150H) was used for sample solution preparation.
Reagents and chemicals
Pure drug sample of DICLO K and THIO were kindly supplied as a gift sample by Zest Pharma, Indore and Glenmark Pharmaceuticals, Sinner,
Nasik, respectively. These samples were used without further purification. Tablet formulation manufactured by Sun Pharmaceutical Industries (Lotensyl, Batch No. AD 92286) was purchased from local market containing DICLO K (50 mg) and THIO (10 mg) per tablet. Spectroscopy grade methanol purchased from Merck, Mumbai was used throughout the study.
Preparation of Standard Stock Solutions and calibration
Curve Standard stock solutions each containing 1000 μg mL-1 of DICLO K and THIO were prepared separately in the methanol. The working standard solutions of these drugs were obtained by dilution of the respective stock solution in methanol. For verification of Beer?s law a series of dilutions in the concentration range of 12.5-62.5 μg mL-1 for DICLO K and 2- 10 μg mL-1 for THIO were prepared in mixture for method A. Solutions in the concentration rang of 25-75 μg mL-1 for DICLO K (series A) and 4-12 μg mL-1 for THIO (series B) were prepared and mixture of both the drugs (series C) in same concentration range was prepared for method B.
Preparation of Sample Stock
Solution For formulation analysis, twenty tablets were weighed accurately and a quantity of tablet powder equivalent to 50 mg of DICLO K (8 mg of THIO) was weighed and dissolved in 40 mL of methanol with the aid of ultrasonicator for 10 min and solution was filtered through Whatman paper No. 41 into a 50 ml volumetric flask. Filter paper was washed with methanol, adding washings to the volumetric flask and volume was made up to mark. The solution was suitably diluted with methanol to get of 37.5 μg/ml of DICLO K and 6 μg/ml of THIO. The proposed methods were followed for analysis.
Procedure
Method A: RATIO DERIVATIVE METHOD
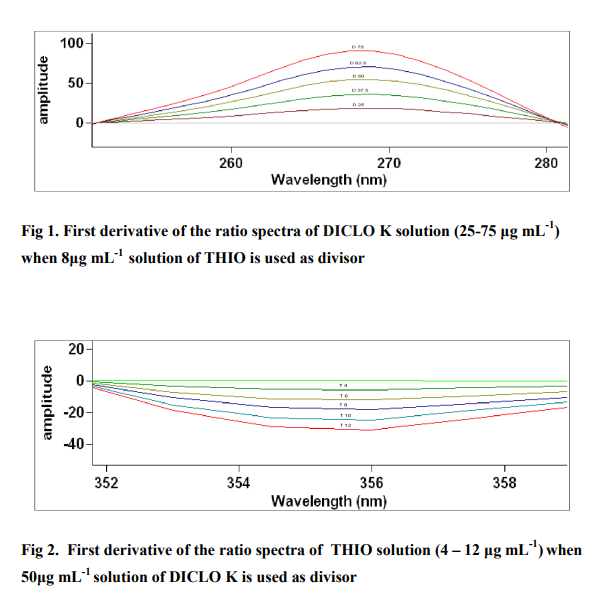
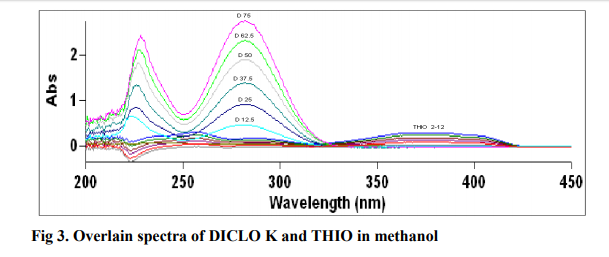
The method involves dividing the spectrum of mixture by the standardized spectra of each of the analyte and deriving the ratio to obtain spectrum that is dependent of concentration of analyte used as a divisor. Using appropriate dilutions of standard stock solution, the two solutions were scanned separately. The ratio spectra of different DICLO K standards at increasing concentrations were obtained by dividing each with the stored spectrum of the standard solution of THIO (6 μg mL-1 ) and the first derivative of these spectra traced, illustrated in Fig 1 . Wavelength 268.78 nm was selected for the quantification of DICLO K in DICLO K + THIO mixture. The ratio and ratio derivative spectra of the solutions of THIO at different concentrations were obtained by dividing each with the stored standard spectrum of the DICLO K (37.5 μg mL-1 ) (Fig. 2). Wavelength 355.62 nm was selected for the quantification of THIO in THIO + DICLO K mixture. Measured analytical signals at these wavelengths were proportional to the concentrations of the drugs. Calibration curves were prepared from the measured signals at the selected wavelength and concentration of the standard solutions. The amount of DICLO K (C DCLO K) and THIO (C THIO) in tablets and capsules was calculated by using equations 1 and 2, respectively. C DICLO K = [Derivative amplitude at 268.78 - (0.0094)] / (1.4434) ... (1) CTHIO = [Derivative amplitude at 355.62 - (-0.0137) ] / (0.0792) ... (2)
Method B: DUAL WAVELENGTH METHOD
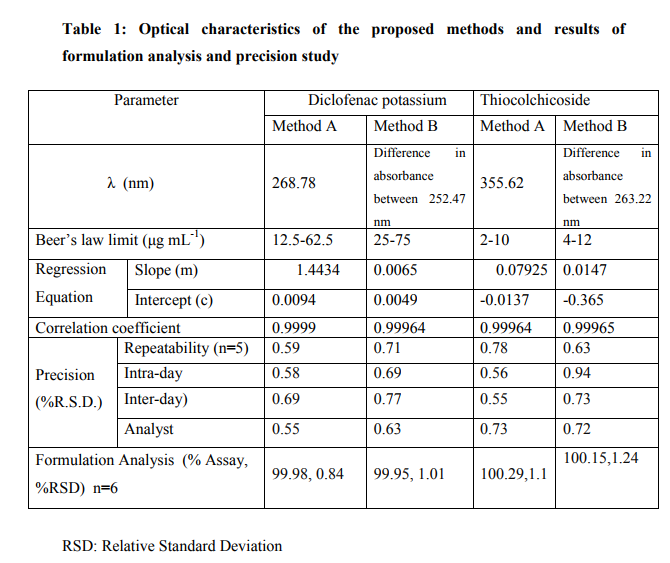
The spectrum of DICLO K shows identical absorbance at 263.22 nm (λ3) and 301.65 nm (λ4) therefore these two wavelengths were selected for the analysis of THIO. All the solutions of series A were scanned to ensure that the difference between λ3 and λ4 is zero. Similarly, the THIO solutions were scanned to determine the two wavelengths, where absorbance is same. These two wavelengths were found to be 252.47 nm (λ1) and 260.74 nm (λ2). All the solutions of series B were scanned to ensure that difference between (λ1) and (λ2) is zero. All the solutions of series c were scanned to verify the results.
Recovery studies
The accuracy of the proposed methods was checked by recovery study, by addition of standard drug solution to preanalysed sample solution at three different concentration levels (50 %, 100 % and 150 %) within the range of linearity for both the drugs. The basic concentration level of sample solution selected for spiking of the drugs standard solution was 37.5 μg/ml of DICLO K and 6 μg/ml of THIO for both the methods.
Precision of the Method
Reparability of the methods was studied by repeating the methods six times. To study intra-day precision, method was repeated 3 times in a day. Similarly the method was repeated on five different days to determine inter-day precision.
RESULTS AND DISCUSSION
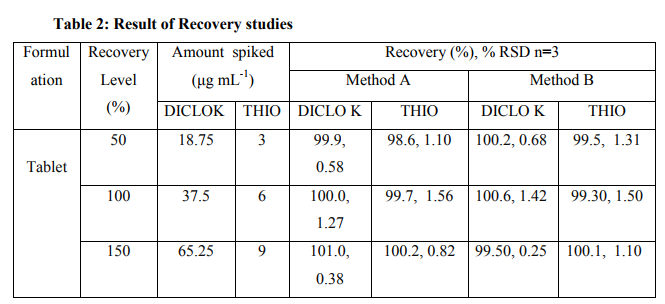
DICLO K and THIO the proposed methods for simultaneous estimation of in combined dosage form were found to be accurate, simple and rapid. The developed methods can be used for routine analysis of two drugs in combined dosage forms. Practically no interference from tablet excipients was observed in these methods. Both the methods are accurate, simple, rapid, precise, reliable, sensitive, reproducible and economical. These methods were validated as per ICH guidelines. The values of % RSD and correlation of coefficient were satisfactory and result of the recovery study indicates that there is no interference due to excipients present in the formulation. Result of formulation analysis and precision study are summarized in table 1 indicated that method is precise. % Recovery (%RSD) for DICLO K by Method A and Method B was found to be in the range of 99.9- 101.0(0.38-1.27) and 99.50-100.6 (0.25- 1.42) respectively and that of THIO by Method A and Method B was found to be in the range of 98.6-100.2(0.82-1.56) and 99.5-100.1(1.10-1.50) (Table 2).
CONCLUSION
The proposed methods are simple, precise, accurate and rapid for the determination of DICLO K and THIO in combined tablet dosage forms. Analysis of authentic samples containing DICLO K and THIO showed no interference from the common additives and excipients. Hence, recommended procedure is well suited for the assay and evaluation of drugs in pharmaceutical preparations. Thus these methods can be easily and conveniently adopted for routine quality control analysis.
ACKNOWLEDGEMENT
The authors are thankful to Zest Pharma, Indore and Glenmark Pharmaceuticals for providing gift samples. The authors are thankful to Management of MAEER?s Maharashtra Institute of Pharmacy, Pune for providing necessary facility for the work.
References:
1. A.H. Beckett, J.B. Stenlake, Practical pharmaceutical chemistry, the Athlone press of university of London, 3rd ed., part two,1976,249.
2. M. Carta *, L. Murru, P. Botta, G.Talani, G. Pietro Sechi, P. De Riu, E. Sanna, G. Biggio, The muscle relaxant thiocolchicoside is an antagonist of GABAA receptor function in the central nervous system, Neuropharmacology 51 (2006) 805-815.
3. W. Schneider, P.H. Degen, Simultaneous determination of diclofenac sodium and its hydroxy metabolites by capillary column gas chromatography with electroncapture detection, Journal of Chromatography A, Volume 217, 6 November 1981, Pages 263-271.
4. Gao, Songmei, Analysis of diclofenac potassium by laser desorption ionization mass spectrum.
5. The Merck index, an encyclopedia of chemicals, drugs and Biologicals, Merck research laboratories, 13th ed., 1997, 3108,9397.
6. A. fini.et al, Thermal and fractional analysis of sodium and potassium salts of Diclofenac.
7. R. K. Prasad*and R.Sharma, Simultaneous estimation and validation of Rabeprazole Sodium and Diclofenac Sodium in capsule dosage form, J. Chem. Pharm. Res., 2010, 2(2): 186-196.
8. Y.K. Agrawal* and K. Shivramchandras, Spectrophotometric determination of diclofenac sodium in tablets, Journal of Pharmaceutical and Biomedical Analysis, Vol. 9, No. 2, 1991, pp. 97-100.
9. N.A. El-Ragehy *, M.M. Ellaithy, M.A. El-Ghobashy, Determination of thiocolchicoside in its binary mixtures (Thiocolchicosideglafenine and Thiocolchicoside-floctafenine) by TLC-densitometry, 58 (2003) 463- 468.
10. Ming-Thau Sheu, Huei-Lan Chou, Ching-Cheng Kao, Cheng-Hsiung Liu, Theodore D. Sokoloski, Dissolution of diclofenac sodium from matrix tablets , International Journal of Pharmaceutics, Volume 85, Issues 1-3, 20 September 1992, Pages 57-63
11. British Pharmacopoeia, London: The Majesty stationary office, 2004; I: 626.
12. M.S. Bhatia, S.R. Dhaneshwar, Simple colorimetric detection of diclofenac sodium from tablets.
13. M.Riegel*, P.P. Ellis, Highperformance liquid chromatographic assay for antiinflammatory agents diclofenac and flurbiprofen in ocular fluids, Journal of Chromatography B, 654 (1994) 140-145.
14. Julio c. Botello and guadalupe pi~rez-caballero*, spectrophotometric determination of Diclofenac sodium with methylene blue, Talanta, Vol. 42, No. I, pp. 105-108, 1995.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License