IJCRR - 3(2), February, 2011
Pages: 42-54
Print Article
Download XML Download PDF
SYNTHESIS AND BIOLOGICAL EVALUATION OF 3,5- DIARYL-1-PHENYL-2-PYRAZOLINES AS ANTIBACTERIAL, ANTI-INFLAMMATORY AND ANALGESIC AGENTS
Author: Anjan Kumar, Sradhasini Rout, Chandrasekar Panda, M.B.V.Raju
Category: Healthcare
Abstract:In a wide search program towards an efficient antibacterial, anti-inflammatory and analgesic
agents, a series of 3,5-diaryl-1-phenyl-2-pyrazoline were synthesized starting from substituted \a,
\? unsaturated carbonyl compounds which undergo cyclization reactions with phenylhydrazine.
The synthesized compounds were characterized and confirmed on the basis of FT-IR, 1HNMR,
13CNMR and mass spectral data. The synthesized compounds 3,5-diaryl-1-phenyl-2-pyrazolines
had shown significant activity against Staphylococcus aureus (MTCC 87), Escherichia Coli
(MTCC 40), Pseudomonas aeruginosa (MTCC 424) and Proteus vulgaris (MTCC 426) by cup
plate method using tetracycline-SD 037 as a reference standard. The anti-inflammatory property
of 1,3,5-diaryl-1-phenyl-2-pyrazolines were screened by using carragenan induced paw edema
method in Wistar rat. The compounds exhibited significant anti-inflammatory property further
evaluated for analgesic activity using both acetic acid-induced abdominal writhing method and
hot plate method respectively in Swiss albino mice. The anti-inflammatory and analgesic
activities exhibited were comparable to that of the standard drug diclofenac. The safety of 3,5-
diaryl-1-phenyl-2-pyrazolines were reflected by toxicity studies.
Keywords: Chalcones; Pyrazolines; Antibacterial; Anti-inflammatory; Analgesic.
Full Text:
INTRODUCTION
Considerable interest has been focused on the pyrazoline structures, which are known to be biologically active and are important constituents of many pharmaceutical1 and agrochemical products2 . As a important class of biologically active agent pyrazoline possesses a broad spectrum of biological activities such as antibacterial3 , antifungal4 , antidepressant5 , antidiabetic6 , antiacetylcholinesterase7 , anti-inflammatory, analgesic8 , antiangiogenic and antioxidant9 . 2- pyrazoline being important nitrogen containing five member heterocyclic compounds seems to be the most frequently studied pyrazoline type compounds. With this background, it is considered worthwhile to synthesize 3,5- diaryl-1-phenyl-2-pyrazolines10 starting from simple condensation of substituted acetophenones with variously substituted aldehydes to give α, β unsaturated carbonyl compounds (2a-j) which undergo a subsequent cyclization reaction with phenylhydrazines affording 2-pyrazolines (3a-j) and screen them for antibacterial, anti-inflammatory and analgesic activities. The mass spectrum of 2a was recorded as additional evidence to the proposed structure. It exhibited molecular ion peak at m/z 208 corresponding to its molecular weight. The other predominant peaks, which were appeared at m/z 178, 105, 89, 77and 51 were in consistent with expected fragmentation pattern. The mass spectrum of 3a was recorded at m/z 298 corresponding to its molecular weight. The other predominant peaks, which were appeared at m/z 220, 180, 105 and 63 were in consistent with expected fragmentation pattern. The chalcones (2a-j) possessing α, β unsaturated ketone group, when reacted with phenylhydrazine underwent condensation and subsequent ring closure to give 2-pyrazolines (3a-j). The involvement of carbonyl group (C=O) in the reaction was indicated by the absence of absorption band at 1658 cm-1 and presence of a characteristic absorption band at 1597 cm-1 due to (C=N) stretching frequency of pyrazoline in the IR spectra of 3a. Similarly chemical shift in 13CNMR reflected at δ 190.63 due to (C=O) group of 2a, which was absent in corresponding 2-pyrazoline rather represented at δ135.57 due to (C=N). The 1HNMR spectrum of 3a-j exhibited two doublets of doublets at δ 2.98-3.17, 3.79-3.95 and 5.20-5.67 due to Ha, Hb of (-CH2) and Hc of (-CH-) proton of 2- pyrazoline respectively. Multiplets were appeared due to aromatic protons with the expected chemical shift and integral value. In present study an attempt had been made to find out the efficiency of inhibition of bacterial growth in gram positive Staphylococcus aureus and gram negative Escherichia Coli, Pseudomonas aeruginosa and Proteus vulgaris, inflammation in Wister Albino rats and pain in Albino mice respectively
.EXPERIMENTAL
Melting points were determined in open capillary tubes are uncorrected. IR spectra were recorded on SHIMADZU FTIR affinity series-I using KBr. and 1HNMR spectra were recorded on BRUKNER AVANCE II 400 NMR SPECTROMETER using CDCl3 as solvent and TMS as an internal standard. Peak values are shown in δ ppm. Mass `2spectra were recorded on AGILENT gas chromatography-mass spectrometer. The purity of the synthesized compounds was checked by TLC on silica gel plates.
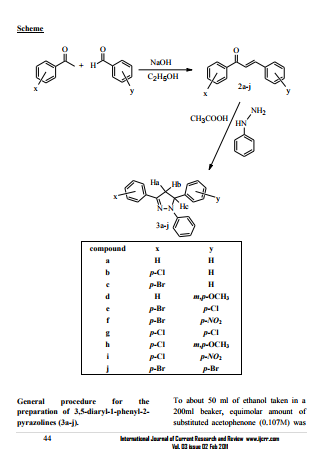
added followed by the addition of substituted benzaldehydes (0.107M) and stirred for 1 hour. About 20ml of (10%) NaOH solution was added slowly to the stirred solution. The mixture was stirred for about 8-10 hours and kept in a deep freeze for an overnight. The solid products 1,3-diaryl prop-2-ene-1-one (2a-j) so formed were neutralized by hydrochloric acid. The products were filtered off and recrystalized by ethanol. After that in a 100ml round bottomed flask, to a solution of compounds (2a-j) (0.025M) in 20ml of glacial acetic acid equimolar of phenyl hydrazine (0.025M) was added, stirred for 1hours and refluxed for 5 hours. The mixtures were kept in deep freeze for an overnight. The solid products 3,5-diaryl-1-phenyl-2- pyrazolines (3a-j) so formed was neutralized by 2% sodium hydroxide solution.The product were recrytallized by ethanol. The completion of the reaction was monitored by TLC.
METHODS
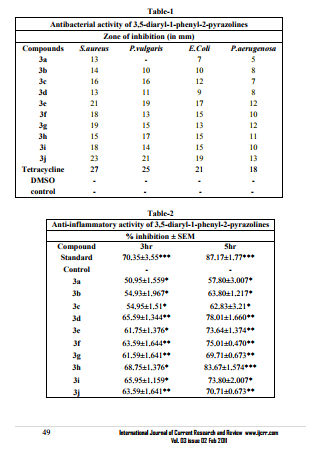
Antibacterial activity The synthesized compounds were screened for in vitro antibacterial activity11 against gram-positive bacteria Staphylococcus aureus (MTCC 87) and gram-negative bacteria Escherichia Coli (MTCC 40), Proteus vulgaris (MTCC 426) and Pseudomonas aeruginosa (MTCC 424) by cup-plate method12 at a concentration 100 µg/ml in DMSO solution using tetracycline-SD 037 as a reference standard. Mueller–Hinton broth was used as medium for antibacterial activity. The Petri dishes were incubated at 36°C for 24 hours. Inhibition was recorded by measuring the diameter of the inhibition zone at the end of the 24hr. Each experiment was repeated duplicate and average of the two independent determinations was recorded. All the 2-pyrazoline compounds showed significant activity against these organisms.
Anti-inflammatory
activity Animals Wistar Albino rats (150-200g) of either sex were used. The animals housed under standard laboratory conditions maintained at 25±1oC and under 12/12 hour light/dark cycle and fed with standard pellet diet (Gold Mohur brand, Lipton India limited.) and water ad libitum. The experimental protocols were approved by Institutional Animal Ethics Committee (Regn No: 926/ab/06/CPESEA).
Acute toxicity study
Acute toxicity of 3,5-diaryl-1-phenyl-2- pyrazoline derivatives were determined in Albino Wistar rats with the staircase method13. Each group of 6 animals was fasted for 24 hour prior to the administration of the test compounds. The test compounds, 3a-j were administered orally in doses up to 2000 mg/kg by suspending in 1% C.M.C solution and were kept under observation for period of 24 hour
Carrageenan induced paw edema The anti-inflammatory activities14 of 3aj were assessed in vitro for their percent inhibition of paw edema in carrageen model of inflammation in albino wistar rats using the method illustrated by Winter et al15. After 16 hours of fast the rats were divided into different groups of six each. Carrageenan (0.1 mL, 1%) was administered into the plantar surface of the right hind paw of the animals. The experimental groups, negative control group (1% CMC), and positive control group (10 mg/kg/po of diclofenac sodium) were given either the control drug and test compounds orally, 1 hour prior to the administration of the carrageenan. Before injection of carrageenan, the average volume (Vo) of the right hind paw of each rat was calculated from 3 readings that did not deviate more than 3%. After injection of the phlogistic agent, the paw volume (Vt) was measured after 1st, 2nd, 3rd, 4th , and 5th hours respectively with the aid of a digital plethysmometer. The edema was expressed as an increase in the volume of paw and percentage inhibition of acute edema was obtained as follows: % inhibition = [1-(?V experimental/ ?V control)] x 100 Where, ?V = Vt-Vo = Mean paw volume
Analgesic Activity Acetic acid induced writhing response (chemical) method
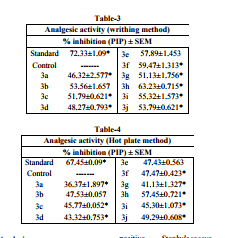
The analgesic activities16 were assessed in vitro for their percent inhibition of nociception in acetic acid-induced abdominal writhing in Swiss albino mice using the method illustrated by Adeyemi et al17. Vehicle, diclofenac sodium and test solution (20 mg/kg/po) were administered orally 30 min before the experiment and 0.1 ml per 10 g of 0.7% acetic acid saline was then injected i.p. 10 min after the injection. The number of writhing during the following 15 min period was counted. The antinociceptive activity will be expressed as percentage inhibition of abdominal writhing was calculated against the control on the basis of experimental data obtained. The pain inhibition percentage (%PIP) was calculated by % PIP = N−Nt / N x 100 N = Average number of stretching of control per group - Nt = Average number of stretching of test per group.
Hot plate (Thermal) method
The hot plate test performed using Eddy’s hot plate maintained at a temperature of 55±1.0 °C described by Turner 18 was used. The mice which showed fore or hind paw licking or jumping response withy in 6-8 secs were selected for the study. After 1 hr of administration of test and reference compounds, the animals of six groups were individually exposed to the hot plate maintained at 55±1.0 °C. A Cut-off period of 15 s was considered as maximal latency to avoid injury to the paws. The time taken by the animals to lick the fore or hind paw or jump out of the place was taken as the reaction time. Diclofenac sodium (20mg/kg/po) was used as a reference drug. The pain inhibition percentage (PIP) was



Statistical Analysis:
Percentage inhibition are expressed in mean ± SEM (n= 6 for each compound). The data were analyzed by One-Way ANOVA, at 99 % confidence interval, followed by Dunnett’s Multiple Comparison Test as post hoc analysis * p < 0.05 Significant Difference, ** p < 0.01 Significant, *** p < 0.001 Highly Significant compared to control and student t-test.
DISCUSSIONS
Antibacterial activity The antibacterial data indicated that the synthesized 3,5-diaryl-1-phenyl-2- pyrazolines (3a-j), showed significant antibacterial activity against gram positive Staphylococcus aureus, moderate activity against gram negative Escherichia Coli, Proteus vulgaris and weak activity against Pseudomonas aerugenosa. It was to note that the compound (3j) carrying p-Br in two phenyl ring at C-3 and C-5 of pyrazoline moiety showed maximum activity followed by compound (3e) carrying pBr and p-Cl in phenyl ring at C-3 and C-5 respectively against all the strains. It was interesting to note that the substitution of electron withdrawing group p-Cl in phenyl at C-3 and electron donating group m,p-OCH3 in phenyl at C-5 led the compound (3h) found to be the good activity as compared to m,p-OCH3 substitution only in compound (3d)against all strains. Detailed antimicrobial activities were tabulated in table-1.
Anti-inflammatory activity
Carrageenan-induced hind paw edema is the standard experimental model of acute inflammation. Carrageenan is the phlogistic agent of choice for testing anti-inflammatory drugs as it is not known to be antigenic and is devoid of apparent systemic effects. The carrageenan test was selected because of its sensitivity in defecting orally active anti-inflammatory agents particularly in the acute phase of inflammation. The percent inhibition of edema was calculated against the control on the basis of experimental data obtained. The percent inhibition was calculated from 1st hour to 5th hour as carrageenaninduced edema is a biphasic response. The first phase (0-3 hrs after injection of carrageenan) is mediated through the release of histamine, serotonin and kinins where as, the second phase is related to the release of prostaglandins and bradykinins. All the 3,5-diaryl-1-phenyl-2-pyrazoline derivatives exhibited encouraging antiinflammatory activity. The oral administration of different derivatives of 2-pyrazoline suppresses inflammation during the both the phase. The compounds showed ranging from 50.95% to 63.59% and 57.80% to 83.67% where as standard drug diclofenac showed 70.35% and 87.17% inhibition after 3rd and 5th hours respectively. Detailed anti-inflammatory activities had given in table-2. The anti-inflammatory activities data showed that compound (3h) having m,pOCH3 and p-Cl groups in the phenyl ring at C-5 and C-3 respectively of pyrazoline nucleus posses highest activity followed by compound (3d) having m,p-OCH3 group in the phenyl ring at C-5. It was noted in compounds (3b) and (3c) that the para substitution of electron with drawing groups of phenyl ring attached to C-3 in pyrazoline moiety shown lesser percentage of antiinflammatory activity. It was interesting to note that when both the phenyl rings at C-3 and C-5 substituted with electron with drawing groups showed significant anti-inflammatory activity. The low toxicity of synthesized compounds was evident from the observation that there was no mortality in rat at doses up to 2000mg/kg. Acetic acid induced writhing response The compounds showing significant anti-inflammatory activity were further screened for their peripheral analgesic activity. The anti-nociceptive activity will be expressed as percentage inhibition of abdominal writhing was calculated against the control on the basis of experimental data obtained. The percent inhibition was calculated after 30 minutes and it was found to be showing significant analgesic activity. Compounds showed analgesic activity ranging from 46.32% to 63.23%, where as standard drug diclofenac sodium showed 72.33% inhibition.
It was observed that compound (3h) showing highest anti-inflammatory activity also exhibited highest analgesic activity 63.23% where as compound (3d) showed decrease in analgesic activity (%), although showed high antiinflammatory activity (%). All other compounds were found to have moderate analgesic activity. Detailed analgesic activities were tabulated in table-3. Hot plate method The compounds showing centrally acting analgesic activity generally elevate the pain threshold of mice towards heat. The highest nociperception of thermal stimulus was exhibited by compound (3h) PIP of 57.45% which is comparable to that of standard diclofenac PIP of 67.45%. Detailed analgesic activities were tabulated in table-4. It was observed that compounds (3e) and (3j) showed significant PIP activities which were earlier exhibited moderate peripheral analgesic activity. It was observed that compound (3d) although exhibited high anti-inflammatory activity (%) showed sharp decrease in analgesic activity (%).All other compounds were found to have moderate centrally acting analgesic activity.
CONCLUSION
The present study focused on potentially active skeleton of 2-pyrazolines. The antibacterial activity of different derivatives of 3,5-diaryl-1-phenyl-2- pyrazoline was investigated by means of cup plate method. It was found that electron with drawing groups (p-Br and/or p-Cl) attached to the phenyl ring at C-3 and C-5 of pyrazoline nucleus showed promising antibacterial activity and it was observed that the compounds 3j and 3e showed highest inhibitory activity against all strains of bacteria. The exact mechanism of action of antibacterial was not performed yet. The oral administration of different derivatives of 2-pyrazoline suppresses inflammation during the both the phase and mechanism of action probably or might be linked to lipoxygenase and/or cycloxygenase. The presence of electron donating group m,p-OCH3 at C-5 and electron with drawing group p-Cl at C-3 of phenyl ring of compound 3h showed maximum inhibitory response as compared to other derivatives and very nearer to standard drug diclofenac. The synthesized compounds had shown good anti-inflammatory were further subjected to analgesic activity and found to be good inhibitor of pain. The abdominal constrictions produced after administration of acetic acid is related to sensitization of nociceptive receptors to prostaglandins. It is therefore possible that the derivatives exert their analgesic effect probably by inhibiting the synthesis or action of prostaglandins. The centrally acting analgesics generally elevate the pain threshold of mice towards heat. In hot plate test 3,5-diaryl- 1-phenyl-2-pyrazolines showed significant pain inhibition percentage. It was interesting to know that the compound 3h which was found to be good anti-inflammatory agent also found to be good inhibitor of pain, which was comparable with the standard drug diclofenac. These synthesized drugs can be extended for further detailed study
ACKNOWLEDGEMENT
Authors are sincerely thankful to Principal and Head of Department of Pharmaceutical Chemistry of Roland institute of pharmaceutical sciences, Berhampur for providing necessary facilities to carrying out the research work.
References:
REFRENCES
1. Gusar N I, Gulko L I, Gorodeskova N R and Klebenov B M. Synthesis and pharmacological activity of 1-aryl-3- amino-2-pyrazoline derivatives. Pharmaceutical Chemistry Journal 1994; 28(4):34-38.
2. Adams J B. Food additive interactions involving sulphur dioxide and ascorbic and nitrous acids. Food Chemistry 1997; 59(3):401-409.
3. Stan D Andrea, Zhizhen Barbara Zheng, Kenneth DenBleyker, Joan C. Fung-Tomc, Hyekyung Yang, Junius Clark, Dennis Taylor, Joanne Bronson. Synthesis and antibacterial activity of dihydro-1,2-oxazine and 2- pyrazoline oxazolidinones: novel analogs of linezolid. Bioorganic and Medicinal Chemistry 2005;5:2834- 2839.
4. Kuntal Manna , Yadvendra K. Agrawal. Microwave assisted synthesis of new indophenazine 1,3,5-trisubstruted pyrazoline derivatives of benzofuran and their antimicrobial activity.Bioorganic and Medicinal Chemistry 2009;19:2688– 2692.
5. Nesrin Gökhan-Kelekci, O Ozgün Simsek, Ayse Ercan , Kemal Yelekçi, Z. Sibel Sahin ,Samil Isik , Gülberk Uçar, A. Altan Bilgin. Synthesis and molecular modeling of some novel hexahydroindazole derivatives as potent monoamine oxidase inhibitors. Bioorganic and Medicinal Chemistry 2009; 17:6761–6772.
6. Pandey V K, Gupta V D and Tiwari D N.Synthesis of Substituted Benzoxazines as potential antiviral agents. Indian Journal of Heterocyclic Chemistry 2004; 13: 399-400.
7. Gulberk Ucar, Nesrin Gokhan, Akgul Yesilada, A. Altan Bilgin.1- N-Substituted thiocarbamoyl-3- phenyl-5-thienyl-2-pyrazolines: A novel cholinesterase and selective monoamine oxidase B inhibitors for the treatment of Parkinson's and Alzheimer's diseases. Neuroscience Letters 2005; 382 (3):327-331.
8. Zafer Asim Kaplancikli, Gulhan Turan-Zitouni, Ahmet Ozdemir, Ozgur Devrim Can, Pierre Chevallet, Synthesis and antinociceptive activities of some pyrazoline derivatives. European Journal of Medicinal Chemistry 200
9. Anand Kumar Tengli, Shrishailappa Badami, B R Prashantha Kumar, Santosh Kumar H Dongre, S Ravi, Durai Ananda Kumar T.Microwave assisted synthesis of pyrazoline derivatives and their antiangiogenic and antioxidant activities. Indian Journal of Heterocyclic Chemistry 2007; (15):333-339.
10. Palaska E, Aytemir M, Uzbay I T and Erol D.Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. European journal of medicinal Chemistry 2001; 36(6):5639-5643.
11. Gruickshank R, Duguid J R, Marmion B P, Swain R H A. Medicinal Microbiology, Vol. 11. Churchill Livingstone, New York; 1975: 190.
12. Minuth J N, Holmes T M, and Musher D M. Activity of Tetracycline, Doxycycline, and Minocycline against MethicillinSusceptible and resistant Staphylococci. Antimicrobil Agent and Chemotherapy 1974; 11:411- 414.
13. Ghosh M N. Fundamentals of experimental Pharmacology. Calcutta, India: Scientific Book agency; 1981:153.
14. Viana G S B, Bandeira M A M, and Amatos F J. Analgesic and antiinflammatory effects of chalcones isolated from Myracrodruon urundeuva Allemao. Phytomedicine 2003; 10: 189–195.
15. Winter C H, Risley E A, Nuss GW. Carageenan induced edema in hind paw of the rat as an assay for antiinfammatory drugs. Proc Soc Exp Bio Med 1962; 111:544-547.
16. Zafer Asim Kaplancikli, Gulhan Turan-Zitouni, Ahmet Ozdemir, Ozgur Devrim Can, Pierre Chevallet. Synthesis and antinociceptive activities of some pyrazoline derivatives. European Journal of Medicinal Chemistry 2009; 44: 2606–2610.
17. Adeyemi O O, Okpo O S and Okpaka O. The analgesic effect of the methanolic extract of Acanthus montanus. Journal of ethnopharmacol 2004; 45.
18. R A Turner. Screening Methods in Pharmacology. New York: Academic Press; 1965. 63.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License