IJCRR - 3(2), February, 2011
Pages: 04-12
Print Article
Download XML Download PDF
SUSTAINED INTRANASAL DRUG DELIVARY SYSTEM FOR THE TREATMENT OF GASTROPARESIS
Author: Agrawal V.A., Rajurkar R.M, Mahle A.M, Thonte S.S.
Category: Healthcare
Abstract:The present investigation concerns about the development of the colloidal dispersion of
mucoadhesive polymers containing Domperidone for intranasal delivery. Colloidal
dispersion was prepared by sonication method and was characterized. Methylcellulose,
Hydroxypropyl methylcellulose and Chitosan were used as a release retardant material.
The nasal isotonic buffer was prepared after calculating the Sodium chloride
displacement (E value) of the ingredients and was used as a vehicle. E values were
calculated from L iso values. The effects of HPMC and Chitosan on drug release
profile were investigated. In the study it was found that HPMC has synergistic effect on
viscosity and bioadhesive strength when used in combination with methyl cellulose.
Formulations were evaluated for in vitro drug release profile, Appearance, pH
examination, Drug content estimation In-vitro Bioadhesion studies at different contact
times, for selection of the best batch. Formulation batch F2 was the optimized and was
further studied for Histopathological examination and accelerated stability.
Keywords: Colloidal dispersion, nasal isotonic buffer, E value, bioadhesion.
Full Text:
INTRODUCTIONThe
idea of bioadhesive polymers to prolong the contact time in the mucosal routes of drug delivery was introduced in the early 1980s and since it has attracted considerable attention by many pharmaceutical scientists. The potential of a drug delivery system to localize a drug at the site of absorption for an extended period and to promote intimate contact between the formulation and the underlying absorbing tissue has a great appeal for both local and systemic effects.1 Mucoadhesive dosage forms have some distinct advantages over conventional dosage forms. This prolongs residential time of dosage forms at the site of application and absorption to permit once or twice a day dosing, localize formulation on mucosa thereby providing a better chance for drug to be absorbed, reduce post nasal drip into the back of the throat and therefore minimize any bad taste problems and loss of drug formulation from nasal cavity, reduces anterior leakage of or drug out of nasal cavity, decrease mucocilliary clearance2 . Gastroparesis the term implies that gastric emptying is delayed in the absence of mechanical obstruction. Gastroparesis3 , also called delayed gastric emptying, is a medical condition consisting of a paresis (partial paralysis) of the stomach, resulting in food remaining in the stomach for a longer period of time than normal. Mucoadhesive colloidal dispersion is a very recent type of formulation for the delivery of insoluble drugs via nasal route. These are the formulations containg single or combination of synthetic, natural or combination of both types of Mucoadhesive polymers in the form of colloidal dispersion prepared by sonication. Generally these are prepared for insoluble drugs when the suspension form of the same drug has less nasal permeation. Domperidone4 is a D2 antagonist, chemically related to Haloperidol, but pharmacologically related to Metoclopramide. Belongs to the class of propulsive i.e. prokinetics. It is a potent antiemetic, antimigraine drug effective for preventing different kinds of emesis and used in gastroparesis. Its conventional dosage form such as tablet and suspension give poor bioavailability (15 to 18%) due to extensive first pass effect, whereas on rapid i.v. injection it has been shown to cause cardiac arrhythmias. Thus to increase its bioavailability (by changing route of administration) and patient compliance Mucoadhesive colloidal dispersion5 was This type of formulation can also solve the problems of conventional nasal drops and water insoluble drugs.
AIM AND OBJECTIVE
The present study was aimed towards preparation of Mucoadhesive colloidal dispersion for intranasal drug delivery which deliver drug in controlled manner and provide a practical, noninvasive and simple method to deliver therapeutic agent. The study was also aimed towards developing such a formulation which improves drug’s solubility characteristics and increases its bioavailability by avoiding First pass effect and gut wall metabolism. The objective of present work was to formulate nasal colloidal dispersion that can prolong contact time with nasal mucosa by mucoadhesion. Avoid First pass effect, reduce dose and dosing frequency by controlling release of drug to evaluate for Bioadhesive strength and Quality control of the preparation.
MATERIALS AND METHODS Materials
-Domperidone was obtained as a gift sample from Ajanta Pharmaceuticals Ltd Mumbai, Methylcellulose A4 M, HPMC K 4 M from ColorconAsia Pvt, Ltd, Goa, Chitosan, Polyethylene glycol 600, Benzalkonium chloride, Sodium chloride and Sodium metabisulphite from Loba chem. Pvt.Ltd.Mumbai, Sodium dihydrogen phosphate and Disodium hydrogen phosphate from Research lab fine chem. Mumbai. Nasal Mucosa was obtained from local slaughter house.
Methods:
Preparation of Standard Curve of Domperidone- Calibration curve for Domperidone in 10% methanolic phosphate buffer of pH 6.4 was prepared. Absorbance was measured using UV visible spectrophotometer (UV 1700Shimadzu) at λ max 284 nm. The graph of absorbance v/s concentration was plotted.
Method of preparation of formulation batches5
: vehicle- Based on the Isotonisity calculation, the amount of NaCl required was found out and Nasal isotonic buffer solution of pH 6.5 was prepared. Polymer phase- The required quantity of polymers was taken and added slowly in 50 ml of vehicle with continuous stirring i mechanical stirrer at 1200 rpm for one hour. It was allowed to swell and hydrate. It was then kept in sonicator for 15 min at 30MHz/sec. to remove any fluff from it. Drug phase- Required quantity of drug and PEG 600 was taken in a glass bottle and 40ml of vehicle was added to it. It was sonicated at 30 MHz/sec. for about 2 hrs. It gives the dispersion of very fine particle size and improved solubility. Mixing of polymer phase with drug phase- The drug phase was added drop wise to the polymer phase udder continuous stirring using magnetic stirrer and mixed for half an hour .The volume was made to 100 ml with vehicle.
Evaluation of formulations
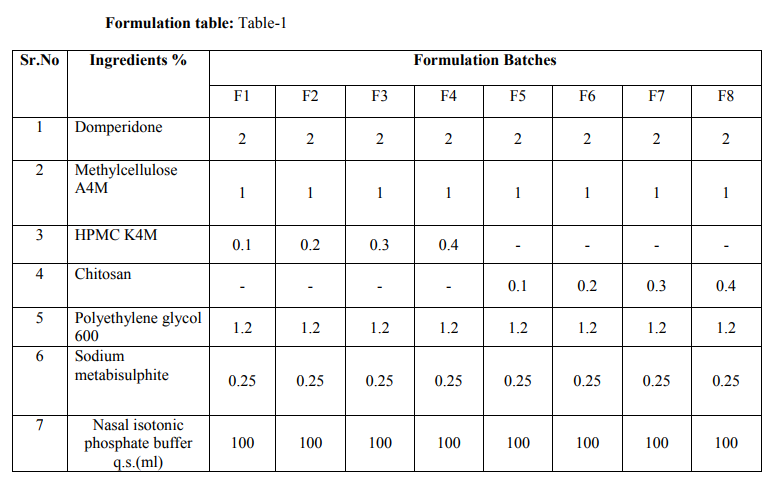
: Appearance: 6 The developed formulation batches were inspected visually. The observations are shown in table no.2.
pH and Density of Mucoadhesive Colloidal Dispersion:7
The pH of each formulation batch was determined by using digital pH meter which was first calibrated by using phosphate buffers of ph 7 and 4. The Density was determined by using specific gravity bottle. The observations are shown in table no2.
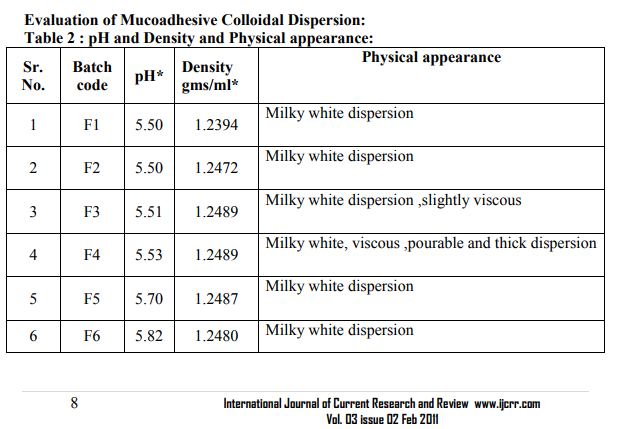
Drug content8 :From each formulation 0.1 ml (equivalent to 2mg) was taken in 100 capacity volumetric flask and diluted with methanolic phosphate buffer pH6.4 up to 100 ml shaken to dissolve. The content of the drug was estimated spectrophotometrically by using standard curve plotted at 284 nm. The observations are shown in table no.3
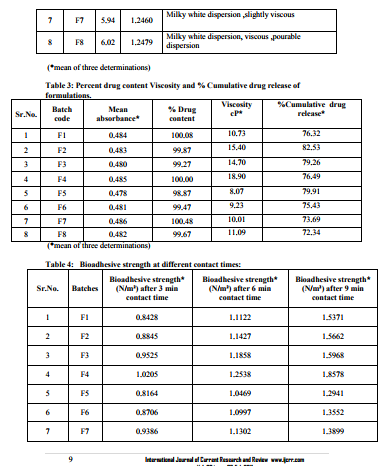
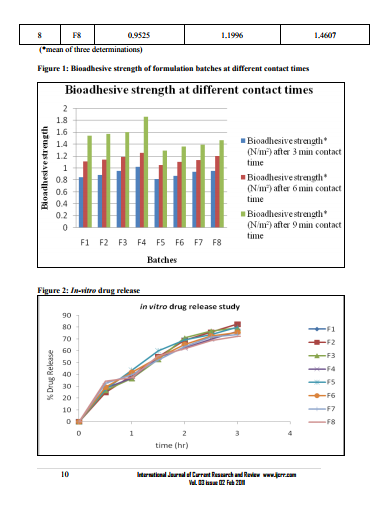
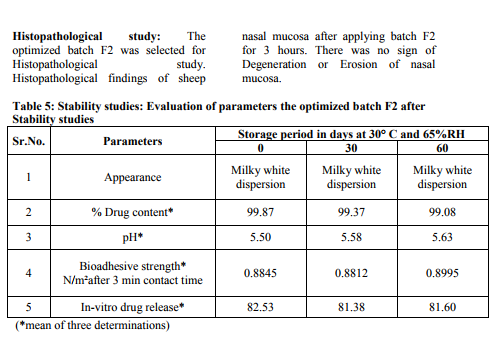
0.1 ml (equivalent to 2mg) was taken in 100 capacity volumetric flask and diluted with methanolic phosphate buffer pH6.4 up to 100 ml shaken to dissolve. The content of the drug was estimated spectrophotometrically by using standard curve plotted at 284 nm. The observations are shown in table no.3. Viscosity measurements -51, 52, 53The viscosities of all the formulations were determined at room temp and at 37 ºC by Brookfield viscometer (DV II pro Model) using spindle no. cP 42 at 20 rpm. The observations are shown in table no.3. Packaging and Pourability: The nasal Mucoadhesive colloidal dispersion was packed in 10 ml capacity HDPE plastic bottles. This packaging system was evaluated for pourability of preparation. Resistance to autoclaving: The teats of packaged system did not become sticky or less resilient and neither the teats nor caps changed in size or shape when autoclaved at 121 ºC and 15 lbs/Sq.inch for 15 minutes, thus the packaged system passed the test of Resistance to autoclaving. Determination of Force of adhesion and Bioadhesive strength 9 The Bioadhesive strength of formulations was evaluated in terms of tensile strength. Freshly excised goat nasal mucosa was used as a model membrane and a lab made assembly was used for the study. This had given the weight in grams required for detachment. Similarly Bioadhesive strength was recorded at different contact times like 6 min and 9 min to evaluate the effect of contact time on Bioadhesive strength of the formulation. In Vitro Release Study:10, 11, 12 In vitro release study of the formulated Mucoadhesive Colloidal Dispersion was carried out in two-chamber diffusion cell through freshly excised goat nasal mucosa. Mucoadhesive Colloidal Dispersion loaded with drug was placed in the donor compartment. 20 ml of 10% methanolic PBS pH 6.4 was placed in the receptor compartment. The temperature of receiver compartment was maintained at the 370C � 1.00C during experiment and the content of the receiver compartment was stirred using magnetic stirrer. An aliquot of 1 ml was withdrawn from receiver compartment at the intervals of 30 min and replaced with same amount of fresh medium to maintain sink conditions. Aliquot so withdrawn were suitably diluted and analyzed using UV spectrophotometer at 284 nm for drug. In vitro drug release study was carried out for 3 hrs. The observations are shown in table no.3. Histopathological study of optimized formulation 13, 14, 15 Mucosa from one nostril was used as control and the other for treatment with optimized batch. After applying Mucoadhesive Colloidal Dispersion for 3 hours the mucosa was fixed in 10% neutral carbonate buffered formalin solution for 24 hours .It was then cut vertically at the central region in 4mm width. Each section was dehydrated using ethanol solutions and was then fixed in paraffin wax. Various sections were taken. These sections were stained with eosin and hematoxylin and were examined for erosion and degeneration using optiphoto light microscope under 60 x resolution power. Accelerated stability study of optimized formulation 16 The formulation batch F2 was stored at temp and relative humidity as given in ICH guidelines for stability testing ofnasal formulations. The storage condition is as mentioned bellow. 30 ºC � 2 ºC and 65 % RH for two months. The samples from the preparation were taken and evaluated for pH, viscosity, drug content, and drug release studies at the intervals of 30 and 60 days. The observations are shown in table no.5.
RESULTS AND DISCUSSION
Statistical parameters for standard curve of Domperidone in 10% Methanolic phosphate buffer pH 6.4- Correlation coefficient (R2 ) = 0.9984Equation for regressed line; Y=0.0248X - 0.0
CONLUSION
In the present study it was found that HPMC shows synergistic effect in the viscosity and bioadhesive strength. pH of all the formulations was found to be in between 5.50-6.02 in the nasal pH range (4.5-6.5), Drug content in between 98.87-100.48 %.Marked increase in viscosity was observed with increase in conc. of HPMC K4M in batches F1 to F4.Chitosan initially increases the diffusion of drug but at later stages decreases it which may be due to its polyelectrolyte and complexing behavior. Batch F2 containing methylcellulose 1 % and HPMCK4M 0.2 %was found to be optimum. The developed formulation can be a better alternative to conventional nasal drops and nasal suspensions by virtue of its rheological properties, ability to enhance bioavailability through its longer residence time and ability to sustain the drug release.
ACKNOWLEDGEMENTS
The authors are very much thankful to Ajanta pharmaceuticals Mumbai for providing me gift sample of drug and Principal Dr. S.S. Khadbadi sir and Prof.U.A. Deokate sir Govt.College of Pharmacy Amravati for providing me Ultrasonic facility.
References:
REFERENCES
1. Haffejee, N., Du-Plessis, J., Mûller, D.G., Schultz, C., Kotze, A.F., Goosen, C. 2001.Intranasal toxicity of selected absorption enhancers. Pharmazie. 56, 882-887.
2. Ahuja, A., Khar, R.K., Ali, J., 1997. Mucoadhesive drug delivery systems. Drug Devliv. Ind. Pharm. 23, 489-515
3. Stanghellini V, Tosetti C, Paternico A, et al. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology 1996; 110:1036– 42.
4. Christopher K Rayner, Michel Horowits (2005) new management approaches for gastroparesis-A review, Nature clinical Practice Gastroenterology and Hepatology 2,454-462.
5. Bhise,S.B, Rajkumar M,Controlled Nasal Delivery of Ondansetron HCl,IJPER,41(3) julsep,2007,pp229-235.
6. Balsuramanium J,Kant S,Pandit J K. in-vitro and in-vivo evaluation of the gelrite gum based ocular delivery system for Indomethacin.ActaPharm 2003;53,251-261.
7. Seveinbjorn Gizurarson,C, C Marriott, Gary P. Martin and Erik Bechgaard, The influence of insulin and some excipients used in nasal insulin preparation on mucocilliary clearance.Int.J.of Pharm. Sci. 1990; 65; 243-247.
8. Charoo A, Kohali K, Ali A. Preparation of in situ forming ophthalmic gels of ciprofloxacin hydrochloride for the treatment of bacterial conjunctivitis; in vitro and in vivo studies. J.Pharm.Sci..2003; 92(2), 407-413.
9. Srividya B, Rita M, Cardoza P D, Sustained ophthalmic delivery of ofloxacin from pH triggered in situ gelling systems. j controlled Rel.2001;73,205-211.
10. Gupta A,Garg S ,S Khar R K,Measurement of bioadhesive strength of mucoadhesive buccal tablets: Design of an in-vitro assembly, Indian Drugs,1992;30(4):152-155
11. Rimi Datta,A K Bandyopadhay, Development of new nasal drug delivery system of diazepam with natural mucoadhesive agent from Trigonella foenun-graecum L,Journal of Scientific and Industrial research,vol.64 Dec.2005,pp 973-977.
12. Maitani Y, Uchida M, Takahashi S, Nagaki NM, Nagai T. Effect of bile salts on the nasal mucosa: Membrane potential measurement. Int J Pharm. 1991; 69, 21-27.
13. Yamamoto T, Maitani Y. Morphologic examination of rabbit nasal mucosa after nasal administration of insulin peanut oil suspension and a powder dosage form with soya bean-derived sterylglucoside. Biopharm Bull. 1995; 18 (6), 887-890.
14. Osth K, Paulsson M, Bjork G, Edsman K. Evaluation of drug release from gels on pig nasal mucosa in a horizontal using chamber. J Control Rel. 2002; 83, 377-388.
15. Carstensen J T. Drug stability: Principle and practice. 2nd Edi, Marcel Dekker, New York, 538- 550, 1995.
16. Singh S. Drug stability test guidelines for international registration of pharmaceuticals. Pharma Times. Aug-1997; 29-42.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License