IJCRR - 3(4), April, 2011
Pages: 69-75
Print Article
Download XML Download PDF
EVALUATION OF ANTIBACTERIAL ACTIVITY OF MORINGA OLEIFERA AGAINST SOME BACTERIAL
STRAINS
Author: Mayee R, Thosar A
Category: Healthcare
Abstract:The chloroform seed extracts of Moringa Oleifera (CSEMO) showed significant antibacterial activity against twenty different gram positive as well as gram negative bacterial strains Staphylococcus aureus 29737, Staphylococcus aureus ML 267, Sarcina luteus 9341, Bacillus pumilus 8241, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 10536, Escherichia coli VC Sonawave 3:37 C, Escherichia coli CD/99/1, Escherichia coli RP4, Escherichia coli 18/9, Escherichia coli K88, Shigella dysenteriae 1, Shigella soneii 1, Shigella soneii BCH 217, Shigella flexneri type 6, Shigella boydii 937, Pseudomonas aeruginosa ATCC 25619, Vibrio cholerae 2,
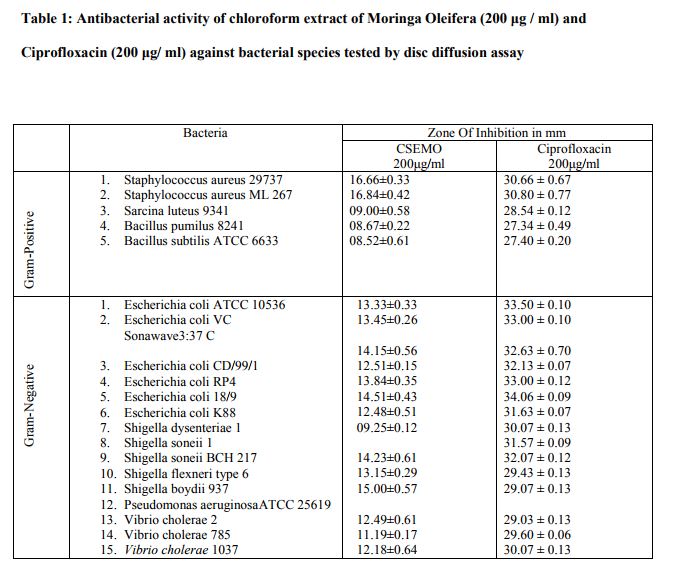
Vibrio cholerae 785, Vibrio cholerae 1037 when compared with Ciprofloxacin (200 \?g/ml) which was used as standard antibacterial by using disc diffusion method.. For gram positive bacteria the CSEMO showed highest sensitivity against Staphylococcus aureus 29737 which was 16.66\?0.33 mm and the lowest activity of 08.52\?0.61 in Bacillus subtilis ATCC 6633. While regarding gram negative bacteria CSEMO showed highest sensitivity against Pseudomonas aeruginosa ATCC 25619 which was 15.00\?0.57 on the other hand it showed minimum activity against Shigella soneii BCH 217 measured as 8.150.10.
Keywords: Moringa Oleifera, antibacterial activity, disc diffusion method, Ciprofloxacin.
Full Text:
INTRODUCTION
The use of medicinal plants as a source for relief from illness can be traced back over five millennia to written documents of the early civilization in China, India and the near east, but it is doubtless an art as old as mankind. Neanderthals living 60,000 years ago in present day iraq used plants such as hollyback, these plants are still widely used in ethnomedicine around the world [1, 2]. The potential of higher plants as source for new drugs is still largely unexplored. Among the estimated 250,000-500,000 plant species, only a small percentage has been investigated phytochemically and the fraction submitted to biological or pharmacological screening is even smaller. Thus, any phytochemical investigation of a given plant will reveal only a very narrow spectrum of its constituents. Historically pharmacological screening of compounds of natural or synthetic origin has been the source of innumerable therapeutic agents. Random screening as tool in discovering new biologically active molecules has been most productive in the area of antibiotics [3, 4]. Even now, contrary to common belief, drugs from higher plants continue to occupy an important niche in modern medicine. On a global basis, at least 130 drugs, all single chemical entities extracted from higher plants, or modified further synthetically, are currently in use, though some of them are now being made synthetically for economic reasons [5]. Medicinal plants represent a rich source of antimicrobial agents. Plants are used medicinally in different countries and are a source of many potent and powerful drugs [6]. A wide range of medicinal plant parts is used for extract as raw drugs and they possess varied Medicinal properties. The different parts used include Root, stem, flower, fruit, twigs exudates and modified plant Organs. While some of these raw drugs are collected in smaller quantities by the local communities and folk Healers for local used, many other raw drugs are collected in larger quantities and traded in the market as the raw material for many herbal industries [7]. Although hundreds of plant species have been tested for antimicrobial properties, the vast majority of have not been adequately evaluated [8]. Considering the vast potentiality of plants as sources for antimicrobial drugs with reference to antibacterial agents, a systematic investigation was undertaken to screen the local flora for antibacterial activity for Moringa Oleifera. Moringa oleifera Lam (Moringaceae), native to the western and sub – Himalayan region, India, Pakistan, Asia Minor, Africa and Arabia [9-10] is now distributed in the Philippines, Cambodia, Central, North and South America and the Caribbean Islands [11]. M. oleifera is a tropical tree whose numerous economic applications and facility of propagation are arousing growing international interest. The Moringa tree is cultivated and use as a vegetable (leaves, green pods, flowers, roasted seeds), for spice (mainly roots), for cooking and cosmetic oil (seeds) and as a medicinal plant (all plant organs) [12]. Moringa oleifera is a highly valued plant, distributed in many countries of the tropics and subtropics. It has an impressive range of medicinal uses with high nutritional value. Different parts of this plant contain a profile of important minerals, and are a good source of protein, vitamins, β – carotene, amino acids and various phenolics [13]. The Moringa plant provides a rich and rare combination of zeatin, quercetin, kaempferom and many other phytochemicals. It is very important for its medicinal value. Various parts of the plant such as the leaves, roots, seed, bark, fruit, flowers and immature pods act as cardiac and circulatory stimulants, possess antitumour [14], antipyretic, antiepileptic, antinflammatory, antiulcer [15]. Other important medicinal properties of the plant include antispasmodic [16], diuretic [11], antihypertensive [17], cholesterol lowering [18], antioxidant, antidiabetic, hepatoprotective [19], antibacterial and antifungal activities [20]. M. oleifera parts are being employed for the treatment of different ailments in the indigenous system of medicine, particularly in South Asia [13]. In addition, M. oleifera seeds possess water purifying powers [21-22] by flocculating Gram – positive and Gram – negative bacterial cells [23-24]. M. Oleifera seeds can also be used as a less expensive bioabsorbent for the removal of heavy metals [25]. The present study was undertaken to investigate the antimicrobial activity of chloroform extract of M. Oleifera seeds
MATERIAL AND METHOD
Plant Material: The plant of M. Oleifera was collected from the roadside locations of Aurangabad (Maharashtra) region and was authenticated by department of Botany, BAMU, Aurangabad. Plant material was preserved in pharmacognosy department of Dr. Ved Prakash Patil, College of Pharmacy, Aurangabad. The leaves were shade dried and powdered in mixer grinder and stored in tightly closed container. Preparation of Extract: The dried powdered seeds (50g) were percolated in 500ml chloroform in 1l capacity conical flasks, stoppered and kept for two weeks with intermittent shaking. The percolate was filtered with Whatman‘s No 1 filter paper. The extract was concentrated at 40o C under reduced pressure using rotary evaporator (R110). In the study of antimicrobial activity, extract was dissolved in Dimethyl sulphoxide (DMSO). The corresponding concentration was expressed in term of μg of extract per ml of solvent (μg/ml). Microorganisms: Twenty different gram positive as well as gram negative bacterial strains namely Staphylococcus aureus 29737, Staphylococcus aureus ML 267, Sarcina luteus 9341, Bacillus pumilus 8241, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 10536, Escherichia coli VC Sonawave 3:37 C, Escherichia coli CD/99/1, Escherichia coli RP4, Escherichia coli 18/9, Escherichia coli K88, Shigella dysenteriae 1, Shigella soneii 1, Shigella soneii BCH 217, Shigella flexneri type 6, Shigella boydii 937, Pseudomonas aeruginosa ATCC 25619, Vibrio cholerae 2, Vibrio cholerae 785, Vibrio cholerae 1037 were collected from microbiology department of Dr. Ved Prakash Patil College of Pharmacy, Aurangabad.
METHOD
The bacterial strains were grown in MacConkey agar plates at 370C and maintained on nutrient agar slants. The antimicrobial activity was performed by disc diffusion assay as per NCCLS, 1993 [26]. The nutrient agar plates containing an inoculum size of 106 cfu / ml for bacteria was used [27].Previously prepared extract impregnated disc (6 mm in diameter) at the concentrations of 200 μg/ml for bacterial was placed aseptically on sensitivity plates with appropriate controls. Ciprofloxacin (200 μg/ml) was used as standard antibacterial. Plates were incubated at 370C for 24 hours for bacteria [28]. Sensitivity was recorded by measuring the clean zone of growth inhibition on agar surface around the disc. The diameters of the inhibition zones were measured in mm.
RESULT
Results obtained in the present study relieved that the tested medicinal plant extract posses potential antibacterial activity against both gram positive- Staphylococcus aureus 29737, Staphylococcus aureus ML 267, Sarcina luteus 9341, Bacillus pumilus 8241, Bacillus subtilis ATCC 6633 as well as gram negative - Escherichia coli ATCC 10536, Escherichia coli VC Sonawave 3:37 C, Escherichia coli CD/99/1, Escherichia coli RP4, Escherichia coli 18/9, Escherichia coli K88, Shigella dysenteriae 1, Shigella soneii 1, Shigella soneii BCH 217, Shigella flexneri type 6, Shigella boydii 937, Pseudomonas aeruginosa ATCC 25619, Vibrio cholerae 2, Vibrio cholerae 785, Vibrio cholerae 1037 bacterial strains (table When tested by the disc diffusion method, the chloroform extract of seeds of Moringa Oleifera showed the highest antibacterial activity for gram positive bacteria of 16.66±0.33 in Staphylococcus aureus 29737 and least activity recorded in Bacillus subtilis ATCC 6633 measured as 08.52±0.61. On the other hand for gram negative bacterial strains the CSEMO showed highest antibacterial activity against Pseudomonas aeruginosa ATCC 25619 measured as 15.00±0.57 while lowest activity seen in Shigella soneii 1 which was 09.25 0.12.
DISCUSSION
The antimicrobial activities of various plants have been reported by many researchers [30, 31]. Phytoconstituents present in plants namely flavonoids, alkaloids, tannins and triterpenoids are producing exciting opportunity for the expansion of modern chemotherapies against wide range of microorganisms [32, 33]. In present study a variety of gram positive, gram negative bacteria stains were selected for the screening of antimicrobial effect of selected plant extract to perceive the antimicrobial spectrum as well to authenticate ethnomedicinal claims. The results of this study showed that the CSEMO have varied antimicrobial activities against the tested organisms. This study has not only shown the scientific basis for some of the therapeutic uses of traditional plants, but also confirmed the ethnomedicinal claims for the selected plants
CONCLUSION
In conclusion, the results of this investigation revealed that chloroform extracts of selected plant possess differentiating antimicrobial activity against selected gram positive as well as gram negative bacterial strains. The differentiating activities against variety of microorganisms of this extracts encourage developing a novel broad spectrum antimicrobial formulation in future.
References:
1. Thomson WAR. Medicines from the Earth, McGraw-Hill Book Co. Maidenhead, United Kingdom. 1978.
2. Stockwell C. Nature‘s pharmacy, Century Hutchinson Ltd, London, United Kingdom. 1988.
3. Gerhartz W, YS Yamamota, FT Campbell, R Pfefferkorn, JF Rounsaville. Ullmann‘s Encyclopedia of Industrial. 1985.
4. Kroschwitz JI, M Howe-Grant. KirkOthmer encyclopedia of chemical Technology. 1992; 2:893. 5. Newman DJ, GM Cragg, KM Snader. The influence of natural products upon drug discovery, Nat Prod Res. 2000; 17: 215-234.
6. Srivastava J, J Lambert, N Vietmeyer. Medicinal plants: An expanding role in development, World Bank Technical Paper. No. 320, 1996.
7. Uniyal SK, KN Singh, P Jamwal, B Lal. Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalayan. J. Ethnobiol. Ethnomed. 2006; 2: 1-14.
8. Balandrin MF, JA Klocke, ES Wurtele, WH Bollinger. Natural plant chemicals: Sources of Industrial and Medicinal materials Science. 1985; 228: 1154- 1160.
9. Somali MA, Bajnedi MA, Al-Faimani SS. Chemical composition and characteristics of M. peregrine seeds and seed oil. J. Am. Chem. Sco. 1984; 61:85 - 86.
10. Mughal MH, Ali G, Srivasta PS, Iqbal M. Improvement of drumstick (M. pterygosperma Gaertn) - a unique source of food and medicine through tissue culture. Harmdad Med. 1999; 42:37 - 42.
11. Morton JF. The horse radish tree: M. pterigosperma (Moringacea). A boon to arid lands, Economic Botany. 1991; 45:318 - 333.
12. Rebecca HSU, Sharon M, Arbainsyah A, Lucienne D. Moringa oleifera: medicinal and socio-economic uses, International Course on Economic Botany, National Herbarium Leiden, Netherlands. 2006: 2 - 6.
13. Farooq A, Sajid L, Muhammad A, Anwarul Hassan G. Moringa oleifera: a food plant with multiple medicinal uses. Phytotherapy Research. 2007; 21:17 - 25.
14. Makonnen E, Hunde A, Damecha G. Hypoglycaemic effect of M. stenopetala aqueous extract in rabbits. Phytother. Res. 1997; 11:147 - 148.
15. Pal SK, Mukherjee PK, Saha BP. Studies on the antiulcer activity of M. oleifera leaf extract on gastric ulcer models in rats, Phytother Res. 1995; 9:463 - 465.
16. Caceres A, Saravia A, Rizzo S, Zabala L, Leon ED, Nave F. harmacological properties of Moringa OleiferaScreening for antispasmodic, antiinflammatoy and diuretic activity. J. Ethnopharmacol. 1992; 36:233 - 237.
17. Dahot MU. Vitamin contents of flowers and seeds of M. oleifera. Pak. J. Biochem. 1988; 21:1 - 24.
18. Mehta LK, Balaraman R, Amin AH, Baffa PA, Gulati OD. Effects of fruits of M. oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J. Ethnopharmacol. 2003; 86:191 - 195.
19. Ruckmani K, Kavimani S, Anandan R, Jaykar B. Effect of Moringa oleifera Lam on paracetamol - induced hepatoxicity. Indian J. Pharm. Sci. 1998; 60:33 - 35.
20. Nickon F, Saud ZA, Rehman MH, Haque ME. In vitro antimicrobial activity of the compound isolated from chloroform extract of M. oleifera Lam. Pak. J. Biol. Sci. 2003; 22:1888 - 1890.
21. Muyibi SA, Evison LM. Optimizing physical parameters affecting coagulation of turbid water with M. oleifera seeds. Water. Res. 1995; 29:2689 - 2695.
22. Kawo AH. Water purification potentials and in-vivo toxicity evaluation of the aqueous and petroleum ether extracts of Calotropis procera (Ait.F) Ait.F. latex and Moringa oleifera Lam seed powder. PhD thesis, Microbiology Unit, Department of Biological Sciences, Bayero University, Kano. 2007: 184.
23. Olsen A. Low technology water purification by bentonite clay and M. oleifera seed flocculation as performed in Sudanese villages: effects on Schistosoma mansoni cercariae. Water Res. 1987; 21: 517 - 522.
24. Broin M, Santaella C, Cuine S, Kokou K, Peltier G, Joet T. Flocculent activity of a recombinant protein from Moringa
oleifera Lam. Seeds. Appl. Microbiol. Biotechnol. 2002; 60:114 - 119.
25. Sharma P, Kumari P, Srivastava MM, Srivastava S. Removal of cadmium from aqueous system by shelled M. oleifera Lam. seed powder. Bioresource Technology. 2006; 97:299 - 305.
26. National Committee for Clinical Laboratory Stamdards (NCCLS), 3rd Ed. approved standard M7-A3, NCCLS, Villanova, PA. 1993.
27. Mandal SC, Nandy A, Pal MP, Saha BP. Evaluation of antimicrobial activity of Asperagus recemosus Willd. Root. Phyto Res. 2000; 14: 118-119.
28. Mandal SC, Majumdar A, Majumdar R. Antibacterial activity of Litsea glutinosa bark. Fitoterapia. 2000; 71: 439.
29. Doughari JH. Antimicrobial activity if Tamarindus indica Linn. Trop J Pharm Res 2006; 5(2): 597-603.
30. Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev 1999; 22: 564-582.
31. Shariff ZU. Modern Herbal Therapy for common Ailments. Nature Pharmacy Series, Spectrum Books Limited, Ibadan, Nigeria in Association with Safari Books (Export) Limited, United Kingdom, 2001; 1: 9-88.
32. Lutterodt GD, Ismail A, Basheer RH, Baharudin HM. Antimicrobial effects of Psidium guajava extracts as one mechanism of its antidiarrhoeal action. Malaysian J Med Sci. 1999; 6 (2):17- 20.
33. Marjorie MC. Plant products as antimicrobial agents. Clin Microbiol Rev 1999; 12(4): 564-582.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License