IJCRR - 3(5), May, 2011
Pages: 04-09
Print Article
Download XML Download PDF
STUDY ON THE EFFECT OF GLYCEROL, PROPYLENE GLYCOL AND POLYSORBATE 80 ON
THE LIPOPHILIC BEHAVIOUR OF TRANDOLAPRIL
Author: Mbah, C. J, Enwereji, P.O
Category: Healthcare
Abstract:The present study examines the lipophilic behaviour of trandolapril by partitioning the drug in a
chloroform-water system at room temperature. The aqueous vehicles investigated are glycerolwater,
propylene glycol-water and polysorbate 80-water systems. The results show that both the
binary combinations and the surfactant solution decreased the lipophilicity of trandolapril. The
order of decrease is: polysorbate 80> propylene glycol> glycerol. The findings suggest that
none of the vehicles investigated, has the potential of enhancing the vehicle-skin partition
coefficient and therefore might not be considered as potential dermal enhancers for trandolapril.
Keywords: Trandolapril, partition coefficient, spectrophotometry.
Full Text:
INTRODUCTION
The desire to use transdermal routes for potent drugs or to avoid certain unsuitable characteristics of oral administered drugs has empowered formulation scientists to employ various formulation approaches with the view of enhancing transdermal drug absorptions. Some of such approaches involve the use of ethosomes, liposomes, microemulsions, nanoemulsions, solid-lipid nanoparticles and transferosomes1,2,3,4 . These nanotechnology-based solutions are lipid-based systems employed to improve drug solubility and bioavailability of poorly soluble compounds. The formulations could contain glycerol, propylene glycol and polysorbate 80 either as co-vehicles or dermal permeation enhancers. For example, propylene glycol has been reported to enhance the dermal permeability of diazepam and midazolam maleate5 , estradiol6 and naloxone7 . Trandolapril (2S,3aR,7As)-1-[(2S)-2-{[(1S)-1- (Ethoxycarbonyl)-3-phenylpropyl]amino}- 1-oxopropyl]octahydro-1H-indole-2- carboxylic acid is a potent nonsulphydryl angiotensin converting enzyme inhibitor. Clinically, it used in the treatment of patients with congestive heart failure, hypertension and myocardial infarction8,9 . Commercially, trandolapril exists only in pharmaceutical solid dosage forms (tablets or capsules), however, the potential of formulating it into transdermal pharmaceutical products exists, if appropriate vehicles (or co-vehicles) and chemical dermal enhancers could be selected. The purpose of this investigation therefore, was to examine the lipophilic behaviour of trandolapril in these vehicles (or co-vehicles) by partitioning the drug between them and chloroform. It is hoped that such information, could provide an insight into its potential dermal absorption. Previous studies10,11.12 have shown that linear correlation exists between permeability coefficient and partition coefficient. Another report13 has also shown dermal permeability coefficient to depend on the partition coefficient and molecular weight of chemical compounds. However, as literature survey has shown little or no study on the lipophilicity of trandolapril in the studied vehicles (or covehicles), in this paper, we report the partitioning of the drug between these vehicles (or co-vehicles) and chloroform
MATERIALS AND METHODS
Materials
Trandolapril (Abbot Laboratories, USA), glycerol, propylene glycol and polysorbate 80 were purchased from Sigma-Aldrich (USA). Chloroform was purchased from Fisher Scientific (USA).
Standard solution
Stock solution of trandolapril (100 g/ml) was prepared in methanol. Aliquots (10.0- 50.0 g/m) of the standard stock solution were pipetted into a 10 ml volumetric flask, diluted to volume with methanol.
Partition coefficient determination.
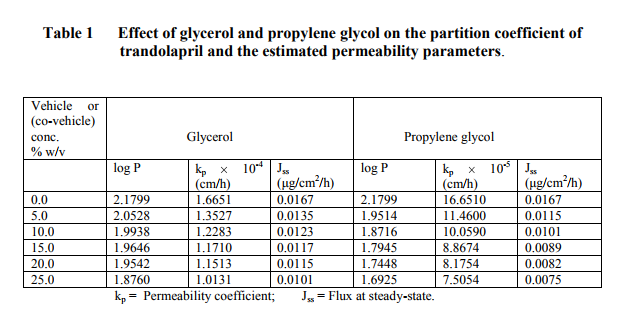
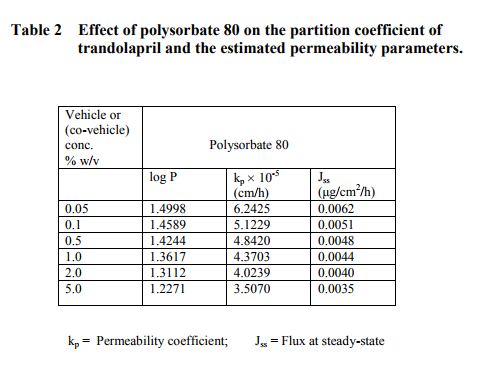
The partition coefficient of trandolapril was determined in a chloroform-water system. To 5 ml of saturated chloroform solution containing 1 mg of trandolapril, 10 ml of saturated aqueous solution of different concentrations of glycerol, propylene glycol and polysorbate 80 were added. The flasks were stoppered and agitated at room temperature for 2 h to achieve complete equilibration. The aqueous phase was analyzed by a spectrophotometric method for trandolapril content at a maximum wavelength of 210 nm and its concentration was calculated from a pre-constructed graph. The partition coefficient of trandolapril was calculated using the following equation14: P = (C1-Cw)Vw CwVo where, P = partition coefficient, C1 = total concentration of trandolapril, Cw = concentration of trandolapril in the aqueous phase, Vw = volume of the aqueous phase, Vo = volume of the organic phase.
RESULTS AND DISCUSSION
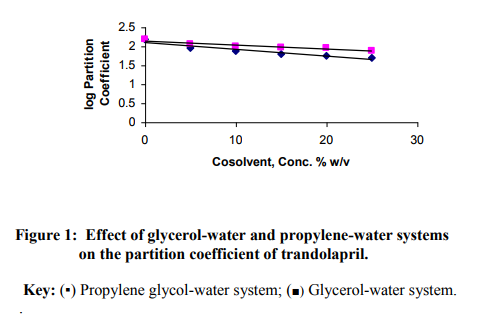
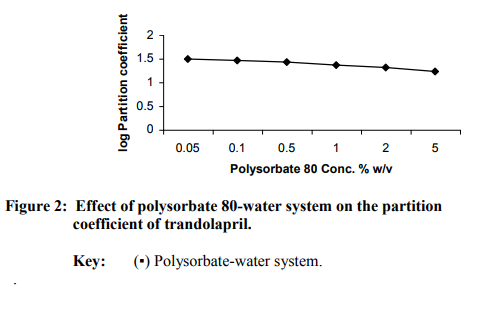
The regression analysis of the calibration graph of trandolapril gave correlation coefficient of 0.9997. The results of the partition coefficient and estimated permeability parameters are presented in Table 1. The results show that all the vehicles or (co-vehicles) decreased the partition coefficient of trandolapril. The decreasing effect was observed with increasing concentration of each vehicle (or co-vehicle). Polysorbate 80-water system was noted to show the highest decreasing effect on the partition coefficient of the drug. The difference between the effect of glycerol and propylene glycol on the partition coefficient of trandolapril could be attributed to decrease in the ability of water molecules to squeeze out the drug molecules from the aqueous environment, resulting in less drug molecules moving into the organic phase. This phenomenon is applicable more to propylene glycol-water system than glycerol-water system. Furthermore, differences in the dielectric constant of the binary systems could also have contributed to the observed difference in the partition coefficient. However, with polysorbate 80-water system, the decrease in the partition coefficient could be as a result of micellar complexation or entrapment of trandolapril in micelles. A plot of logarithm of the observed partition coefficient versus the concentration of vehicle (or co-vehicle) gave linear relationships with correlation coefficients of –0.9501, –0.9498 for glycerol-water and propylene-water systems respectively and – 0.9399 polysorbate 80-water system. The graphs are shown in figure1 for the binary systems and figure 2 for polysorbate 80- water system. As an earlier report15 has shown that permeability coefficient is a useful parameter in evaluating percutaneous absorption, the potential permeability coefficient of trandolapril through the skin was estimated using the observed partition coefficient. The estimation was done by the application of previously reported dermal permeability equation13: log kp (cm/h) = -2.7 + 0.71 log P – 0.0061 MW, where kp is the dermal permeability coefficient, P is the observed partition coefficient of trandolapril, MW is the molecular weight of trandolapril. The estimated permeability coefficients were used to calculate the potential fluxes at steady-state using the following equation16: kp = Jss/C, where Jss is the flux at steadystate, C is the concentration (μg / ml) of the test compound. A comparison of the kp values of trandolapril in glycerol-water; propylene glycol-water system and polysorbate 80-water systems at a concentration level of 5 % w/v, showed glycerol-water system (1.3527 10-4 cm/h) with factors of about 1.2 and 3.9 much greater than propylene glycol-water system (11.460 10-5 cm/h) and polysorbate 80-water system (3.5070 10-5
CONCLUSION
The investigation shows that the studied vehicles (or co-vehicles) decreased the partition coefficient of trandolapril. Polysorbate 80-water system was observed to produce the highest decrease in the lipophilicity (defined by the partition coefficient) of trandolapril. As permeability through the skin depends partly on the partition coefficient of the drug, the study suggests that none of the vehicles (or covehicles) could enhance the transdermal absorption of trandolapril through vehicle/skin partition coefficient mechanism.
References:
REFERENCES
1. Touitou E, Godin B, Dayan N, Weiss C, Piliponky A, Levi-Schaffer F. Intracellular delivery mediated by an ethosomal carrier. Biomaterials 2001; 22:3053-59.
2. Baboota S, Alazaki A, Kehl K, Ali J, Dixit N, Shakael F. Development and evaluation of a microemulsion formulation for transdermal delivery of terbenafine. PDA J. Pharm. Sci. Technol. 2007; 61: 276-85.
3. Shakael F, Baboota S, Ahuja A, Shafq S, Faisal S, Ali J. Enhanced transdermal delivery of aceclofenac using nanoemulsion technique. J Pharm Pharmacol 2007; 93: 31-7.
4. Shafiq S, Shakael F, Telegaankar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007; 66: 227-42.
5. Toniton E. Transdermal delivery of anxiolytics. In vitro skin permeation of midazolam maleate and diazepam. Int J Pharm 1986; 33: 37-43.
6. Mollsaard B, Hoelgaard A. Permeation of estradiol through the skin. Effect of vehicles. Int J Pharm 1983;15: 185-97.
7. Panchagnula R, Salve PS, Thomas NS, Jain AK, Ramarao P. Transdermal delivery of naloxone: effect of water, propylene glycol, ethanol and their binary combinations on permeation through rat skin. Int J Pharm 2001; 219:95-105.
8. Peters DC, Noble S, Plosker GL. Trandolapril: An update on its pharmacology and therapeutic use in cardiovascular disorders. Drugs 1998; 56: 871-893.
9. Pederson OD, Bagger H, Kober L, Pederson C. Trandolapril reduces the incidence atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation 1999; 100: 376-80.
10. Hatanaka T, Inuma M, Sugibayashi K, Marimoto Y. Prediction of skin permeability of drugs 1: Comparison with artificial membrane. Chem Pharm Bull 1990; 38:3452-59.
11. Bunge AL, Cleck RL. A new method for estimating dermal absorption from chemical exposure 2, Effect of molecular weight and octanol-water partitioning. Pharm Res 1995; 12:88- 95.
12. Wagner H, Kosita K, Lehr C, Shaefer U. Correlation between stratum corneum/water partition coefficient and the amounts of flufenamic acid penetrated into the stratum corneum. J. Pharm. Sci. 2002; 91: 1915-21.
13. Potts RV, Guy RH. Predicting skin permeability. Pharm Res 1992; 9: 663-669.
14. Johnansen M, Bundgaard H Prodrugs as drug delivery systems XII. Solubility, dissolution and partition behaviour of N-mannich bases and N-hydroxymethyl derivatives. Arch Pharm Chem Sci Edu 1980a; 8:141-45.
15. Korinth S, Schaller KH, Drexler H. Is permeability coefficient (Kp) a reliable tool in percutaneous absorption studies. Arch Toxicol 2005; 79:155-59.
16. Julreht K, Keith AP, James AW. Development of a transdermal delivery device for melatoin in vitro studies. Drug Dev Ind Pharm 1998; 21; 1377-87.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License