IJCRR - 3(9), September, 2011
Pages: 134-140
Print Article
Download XML Download PDF
BIODEGRADATION OF METHYLENE BLUE BY ENTEROBACTER SP. FROM PALK STRAIT, SOUTHEAST
COAST OF INDIA
Author: Govindasamy. C, Ruban. P, Arulpriya. M.
Category: General Sciences
Abstract:Methylene blue is a heterocyclic aromatic chemical compound; it has many uses in a range of different fields, such as biology, chemistry, textile industries, etc. Colour stuff discharged from these fields poses certain hazards and environmental problems. Marine sediment samples were collected from Palk Strait, southeast coast of India. A total of 10 morphologically different bacterial strains were isolated and enrichment method using as screening medium supplemented with methylene blue (0.01%). All the strains were tested by their ability to degrade methylene blue at different concentrations (10 -150 mg/ml of screening medium). Of these ten isolates, strain M10 degraded maximum of 100 mg/ml of methylene blue (plate method). The potential strain was identified as gram negative, rod shape, non-motile bacteria. Based on the biochemical test it may be confirm as Enterobacter sp. This bacterium inoculated in Methylene blue, Malachite Green, Eosin Yellow dyes in different concentration, the percentage of degradation after 48 hours viz., 67.30%, 53.01%, and 72.30%. Present investigation, resulted that rapid microbial degradation of textile dyes this type of bacterium may be degrade the any type of synthetic dyes
in variable and extensively may be used in the textile industry.
Keywords: Biodegradation, Marine Sediment, Methylene blue, Malachite Green, Eosin Yellow, Enterobacter sp.
Full Text:
INTRODUCTION
Dyes are organic compounds used as coloring agents in chemical, textile, pulp and paper, printing, cosmetic, leather and food industries (Gulanz et al., 2004; Kumar and Porkodi, 2007). In process of washing and finishing colored products, waste water contaminated with dyes is generated. The contaminated waste waters are hazardous which is great to threat to environment (Zhao et al., 2008; Ugurlu, 2009). The removal of textile dyes from waste water is a one of the most important environmental issues to be solved today discharge of hazardous waste water without additional treatment can seriously damage the environment. The colored discharged effluents inhibit penetration of sunlight and oxygen which are crucial requirements of aquatic life (Vadivelan et al., 2007). Biodegradation of dyes is not an easy process due to their toxic and complex aromatic structure (Ozer et al., 2007 ;Yasin et al.,2007). In typical dyeing processes, 50-100% of the dye is fixed on the fiber, and the unfixed dyes are discharged in spent dye-baths or in the wastewater from subsequent textile-washing operations (Jiraratananon et al., 2002). Methylene blue (MB) is commonly used in textile and paper industries it may deleteriously affect humans, fish, algae, and submerged plants in various ways Bana et al.,2007; Nilratnisakorn et al., 2008). MB is a heterocyclic aromatic compound with the molecular formula C16H18ClN3S. At room temperature, it appears as a solid, odorless, dark green powder, which gives a dark blue color when dissolved in water. Various methods, such as membrane filtration, ion exchange, chemical coagulation, oxidationreduction, and adsorption onto activated carbon, are available for removing or decolorizing dyebearing wastewaters. However, these methods are very expensive (Crini, 2006; Kumaret al., 2007). The utilization of alternative low-cost materials with high adsorption activity to solve environmental problems has received considerable attention over the recent years.
MATERAIAL AND METHODS
Chemicals (Dyes)
Methylene Blue (MB), Malachite green, Eosin yellow was obtained from Sisco Research Laboratories Pvt Ltd, Mumbai, India. The chemicals used were of the highest purity available and of an analytical grade.
Sediment Sample Collection
Present study, for the isolation of dye degrading bacteria, soil samples were collected from Thondi cost, southeast cost of India. Sediment samples were collected in the clean polyethylene bags and transported to the laboratory by keeping them in ice box and processed within 3 hours and microbial analysis were carried within 4 hours.
Screening of dye degrading bacteria
5 grams of sediment sample was diluted to 50% seawater and homogenized thoroughly and store at 40C. Simultaneously screening agar medium was prepared supplemented with Methylene blue at minimal concentration (0.01%). After preparation 0.1ml of sample were inoculated into the plate and spreaded sterile L-road. After then all the plates were incubated at room temperature for 48-72hrs. One uninoculated plate was kept as a control.
Screening Medium Composition (Chen et al., 2003)
Yeast extract-10gm/lit; NaCl- 5gm/lit; Peptone- 5g/lit; Agar-10gm/lit; Methylene Blue- 0.1gm/lit; pH-7.0.
Recovery and preparation of Stock Culture
After inoculation, morphologically different colonies were observed on screening medium and then colonies with zone of clearance on screening medium were selected as dye degrading strains (Chen et al., 2003). All the selected colonies were transferred into nutrient agar plates. After the inoculation all the strains were subcultured and maintained as stock culture on nutrient agar slants for further.
Degrading of Multi-dye degradation by potential strain in shake flask method
The screening medium was prepared with increasing concentration of Methylene blue, Malachite green, Eosin yellow (10 to 150mg/100ml) in conical flask. After the sterilization 1 ml broth culture of potential strain was inoculated and placed on rotary shaker at 120 rpm for 24-48 hours at room temperature.
Assay of Methylene Blue decolorization by the potential strains: (Suneja et al., 2004)
Decolorization was expressed in terms of percentage of decolorization. Dye decolorization by the potential isolates in the broth cultures was assayed by using colorimetric method. Prior to this, the absorption maximum of the Methylene blue supplemented broth was determined. The absorption maxima were 540 nm for dye incorporated broth and hence decolorization was assayed at 540 nm. The culture broth was centrifuged at 1000rpm for 10 minutes in and the bacterial cells were removed in pellets. The supernatant was analyzed calorimetrically at 540nm to measure the percentage of dye decolorization. Then the percentage of dye degradation was calculated by applying the absorbance value below formula described by Sani and Banerje, 1999.

Identification of potential strain Phenotypic characteristic such as microscopic appearance (grams staining, motility), cultural and biochemical characteristics of potential strain was studied by adopting standard procedures. Then the potential strain was identified based on the studied phenotypic properties.
RESULTS AND DISCUSSION
Synthetic dyes are artificially produced by which a large group of organic chemicals which encountered in practically all spheres of our daily life. In this the usage of organic chemical may be have on undesirable effect not only on the human being as well as environment, in this correlation several researchers has studied about the many physical and chemical methods, including adsorption, photodegradation, precipitation, filtration and processing units have been used for the treatment of dye-containing effluent. Currently, microbial biodegradation has become a promising approach or dye treatment because it is cheaper effective and more environmentally friendly but this study related with biological degradation by using microbial culture is to restricted (Zhou and Zimmermann, 1993). In view of this the present study was made on attempt to identify the possible biodegradation of industrial dyes by using marine associated bacterial isolates. The colony morphology was observed after five days of incubation total of ten morphologically different colonies were observed on isolation medium. A clear zone of decolorization was observed around the colonies and named as strain M1, M2, M3 till M10. All the strains were recovered using nutrient agar plates and transferred to the nutrient agar slants as stock for further studies. After the incubation of screening medium plates with increased concentration of selected dye Methylene blue the following results were obtained (Table 1)
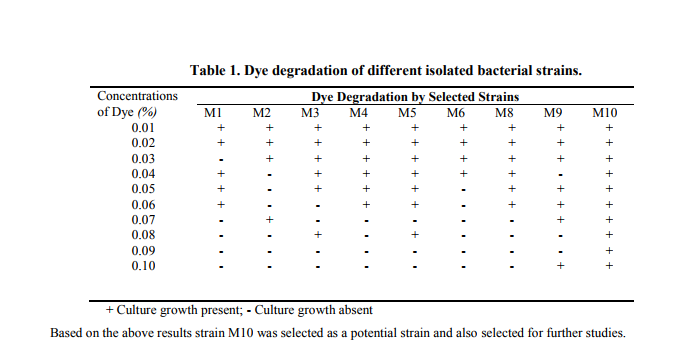
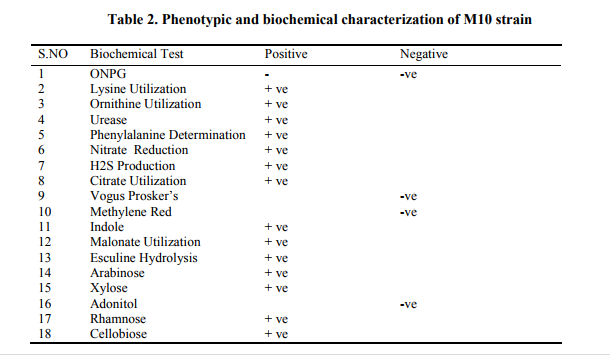
Characterization and identification of potential strain
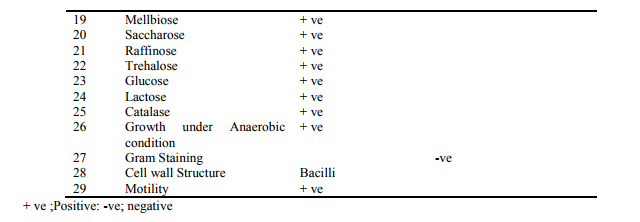
There are many methods for identifying bacteria. Traditionally an observational and biochemical approach has been used. Simply looking at a bacterial colony growing on an agar plate can give an experienced researcher clues to a bacterium's identity. Bacteria are categorized as "Gram Positive" or "Gram Negative" according to whether or not they are stained by a chemical dye, a common biochemical techniques (Mahalakshmi et al., 2011). All the phenotypic and biochemical characteristics of strain M10 were given in Table 2. Based on the characteristics the Potential strain M10 was comes under Enterobacter sp.


Degradation of Multi-dyes by potential Strain M10 flask method and assay of decolorization
There are many reports on decolorization of textile dyes in flask method by microorganisms (Suneja et al., 2004). Present experiments focus, after incubation of screening medium with increased concentration of Methylene blue, Malachite Green, Eosin Yellow in shake flask method, the following results were obtained. From this observation, the strain M10 can able to degrade at the maximum viz., 34.29%, 40.12%, 54.50% in dye (0.13) concentration. But the percentage of degradation was decreased when increase the concentration of dyes (Table. 3). In optimum pH 7.0 the Enterobacter sp. was degraded Methylene blue, Malachite Green, Eosin Yellow the percentage viz., 67.30%, 53.01%, 72.30% (Table. 4). The results suggest that the optimum pH for the biodegradation of methylene blue, malachite green, and eosin yellow was degraded maximum at the pH of 7 that may be due to the bacteria and caused release of enzymes or redox mediators to cause dye reduction, or the dye may be reduced by alkaline hydrolysis.


The decolorization mechanism of Enterobacter sp. involved dye adsorption and dye reduction. Its decolorization depends on dye concentration, glucose, and pH of the dye medium (Forgacs et al., 2004). These bacteria may decolorize the dye under reduction either by electron transport from the cellular metabolic pathways of flavins or quinines, or by external redox mediators as well as by azoreductase enzymes (Pandey et al., 2007) Therefore, Enterobacter sp. was the best decolorizer It may remove dye by initial adsorption together with decolorizing by electron transport under aerobic conditions and by azoreductase enzymes. It‘s concluded from the presence findings that the isolated Enterobacter bacterial species from the sediment samples, Thondi cost southeast cost of India showed potential biodegradation effect on textile dye degradation. Further studies are highly required to identify the chemical constitution for the biodegradation is highly necessary.
CONCLUSION
The present study revealed the ability of Enterobacter sp. to decolorize Methylene blue, Malachite Green, and Eosin yellow. Results obtained this work showed that this Enterobacter sp. possessed high decolorization efficiency against multi-dyes. In conclusion this type of bacterium may be degrade the any type of synthetic dyes in variable and extensively may be used in the textile industry.
ACKNOWLEDGEMENT
We thank Alagappa University and the University authorities for facilities and encouragement
References:
1. Banat, F., Al-Asheh, S., Al-Ahmad, R. and Bni-Khalid, F. 2007. Bench-scale and packed bed sorption of methylene blue using treated olive pomace and charcoal, Bioresour. Technol., 98, 3017–3025.
2. Chen, K.C., Wu, J. Y., Liou D. J. and Hwang, S.C.J. 2003. Decolorization of textile dyes by newly isolated bacterial strains. J. of biotechno., 101, 57-68.
3. Crini, G. 2006. Non-conventional low-cost adsorbents for dye removal: A review, Bioresour. Technol., 97, 1061–1085.
4. Gulanz, O., Kayar, A. and Arikan, B. 2004. Sorption of basic dyes from aqueous solution by activated sludge. J. Hazarard Mater., 108, 183-188.
5. Kumar, K. V. and Porkodi, K. 2007. Mass transfer, kinetics and equilibrium studies for the biosorption of methylene blue using Paspalum notatum, J. Hazard. Mater., 146, 214–226.
6. Mahalakshmi. M., Srinivasan, M., Murugan, M., Balakrishnan, S. and Devanathan. K. 2011. Isolation and identification of total heterotrophic bacteria and human pathogens in water and sediment from Cuddalore fishing harbour after the tsunami. Asian. J. Biolol. Scie., 4(2),148- 156.
7. Nilratnisakorn, S., Thiravetyan, P. and Nakbanpote, W. 2007. Synthetic reactive dye wastewater treatment by narrow-leaved cattails (Typha angustifolia Linn.): Effects of dye, salinity and metals, Sci. Total Environ., 384, 67–76.
8. Ozer, D., Dursun G. and Ozer, A. 2007. Methylene blue adsorption from aques solution by dehydrated peanut hull. J. Hazard. Mater., 144, 171-179.
9. Jiraratananon. R., Sungpet. R. and Luangsowan, P. 2002. Performance evaluation of nanofilteration membranes of effluents containing reactive dye and salt, Desalination, Water Resear., 130, 177-183.
10. Sani, R. K. and Banerjee, U.C. 1999 Decolourization of triphenyl methane dyes and textile dye stuff effluent by Kurthia sp. Enzy. Microbiolol. Technol., 24 (7), 433- 437.
11. Suneja, S., Kukreja, R., Gera, R., Yadav, K. S. and Laxshminarayan, K. 2004. Decolorization of triphenlmethane dyes by Azotobacter Chroococcum. J. Poll Res, 23, 643-647.
12. Ugurlu, M. 2009. Adosrption of a textile dye onto activated sepiolite. Microporous and Mesoporous mater., 119, 276-283.
13. Vadivelan, V. and Kumar, V. 2005. Equilibrium, kinetics, mechanism and process design for the sorption of Methylene blue on to rice husk. J. Colloid interface Sci., 286, 90-100.
14. Yasin, M., Hussein, M. Z. and Ahmad, F. H. 2007. Adsorption of Methylene blue onto treated activate carbon. The Malaysian J. Analytical Sci., 11, 400-406.
15. Zhao, M., Tang Z. and Liu, P. 2008. Removal of Methylene blue from aqueous solution with silica nano-sheets derived from vermiculate. J. Hazardous Materials., 158, 43-51.
16. Zhou, W. and Zimmermann, W. 1993. Decolorization of industrial effluents containing reactive dyes by actinomycetes. FEMS Microbiol. Lett.,. 107, 157–162.
17. Forgacs, E., Cserhi. T. and Oros. G. 2004. Removal of Synthetic dyes from Wastewaters: a Review. Environ. Int. 30, 953-971.
18. Pandey, A., Singh. P. and Iyengar. L. 2007. Bacterial Decolorization and Degradation of Azo dyes. Int. Biodeter. Biodegr., 59, 13-84.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License