IJCRR - 3(9), September, 2011
Pages: 66-76
Print Article
Download XML Download PDF
THE INTERACTIONS OF PH, ORGANIC MATTER AND SOIL MOISTURE ON ARBUSCULAR MYCORHIZAL FUNGI (AMF) COLONIZATION IN THE ROOTS OF Juniperus procera IN SAUDI ARABIA
Author: AL-Ghamdi, A. A. M., Hasnah M. J.
Category: General Sciences
Abstract:The symbiosis between AMF and wild Juniperus procera was studied in the soil of Al-Baha, Saudi
Arabia. In this study, the effect of soil pH, moisture and organic matter (OM) on the colonization of AMF was investigated. J. procera infection by AMF was of the Paris type in which coarse hyphae occupied inter- and intracellular cortical cells. The spores in the soil were identified as Glomus sp, Gigaspora sp. and Acaulospora sp. Percent root colonization with AMF was positively related to pH value (r2= 0.81) and moisture value (r2= 0.75). Nevertheless, AMF colonization in the roots was lower with higher OM content (r2= 0.80). It is important to ensure that soil conditions and properties are maintained in such a way that encourages the growth of AMF and the plant. The use of AMF may be an important consideration in the replanting of this endangered plant species in the future.
Keywords: Arbuscular mycorrhiza fungi; soil pH; OM; soil moisture; Juniperus procera
Full Text:
1. INTRODUCTION
Plants living in arid to semiarid ecosystems have developed various ways to combat drought stress (Bray, 1997). One of the most ancient and common plant strategies is the symbiosis of plant roots with AMF so as to improve survival under environmental stress (Brachmann and Parniske, 2006). Although not much is known on the effects of AMF on the water relations and moisture withholding capacity of soils, soil with AMF was found to have considerably more aggregates that are water stable (Augé, 2004). According to Miransari et al. (2008), a vast network of hypha and organic matter of elevated quantities are generated by AMF. In Saudi Arabia, the current population of J. procera only represents a small fragment of the woodlands that were once present (Negash, 2002). According to Hajar et al. (1991) and Al-Gamdi (2006), juniper forests in the southwest region of Saudi Arabia are exposed to more risks of deterioration because it has low natural regeneration capability and there is a lack of plantation for regeneration. Juniper trees at very low altitudes are also diminishing as a result of soil disturbances and the act of extracting young plants from juniper stands (Hajar et al., 1991). The hypothesis of this study is AMF infection is abundant in an arid environment of Saudi Arabian soil. There was scarcely any study done on the colonization of AMF in Juniperous procera and its relationship to the physical property of soil. This study was undertaken to determine the presence of AMF in the soil and roots and to study the relationship between AMF in the roots of J. procera in the southwest of Saudi Arabia Kingdom and soil pH, OM and moisture which can indirectly affect the growth and development of the trees.
2. MATERIALS AND METHODS
2.1 SOIL AND ROOT HAIRS SAMPLE COLLECTION
Soil and root hairs sample were collected from four areas located in AL-Sarawat (AL-Janabin, Athroub, Shakr, and Hazna) with coordinates ranging from latitude of N 19° 05' to 19° 53' and longitude of E 41° 32' to 41° 43'. At intervals of 20 m, six subareas were selectively chosen. At depths of about 30 cm, five soil samples which also include the roots were randomly collected from each subarea. The roots of J. procera trees were also removed of any soil debris and collected into plastic bags to retain its moisture. There were a total of 30 samples per area and the total soil sample for each area was 30 kg. The soil and root hairs were then brought back to the laboratory for further analysis. The soil samples were subjected to pH, OM and moisture analysis, while the roots and soil were examined for AM colonization.
2.2 CLEARING AND STAINING OF ROOT HAIRS
Clearing and staining of root hairs were done according to the methods stated by Brundrett et al. (1996) by using hydrochloric acid (HCl), 0.1%, lactoglycerol, Trypan blue stain (0.05%) and potassium hydroxide (KOH) (10%). First, the root hairs were washed with water to remove any soil particles and then treated with 10% KOH (w/v) until the root hairs were sufficiently cleared. Then, the root hairs were placed in an autoclave for about 15-20 minutes at 121°C. The roots were then rinsed with water and further washed with 0.01% HCl. Before the root hairs were transferred into the staining solution (Trypan Blue 0.05%), the roots were again rinsed with water (Brundrett et al., 1996). Acidified root hairs were left overnight in the solution to be stained. Next, the stained roots were removed from the staining solution and destained with lactoglycerol. Microscopic observations were done after the roots were destained. Stained fungal structures can now be easily recognized.

2.3 SPORE ISOLATION AND IDENTIFICATION
According to the method proposed by Brundrett et al. (1996), AMF spores in 50 g of soil samples were isolated by the wet sieving and decanting method and subsequently subjected to sucrose centrifugation. The supernatant after centrifugation was further sieved using a 50 um sized sieve, rinsed with water and filtered through a filter paper. Three replicates were carried out for each soil sample. Isolated spores based on morphological characters were placed on slides and stained with a mixture of Melzer‘s reagent and polyvinyl alcohol- lactoglycerol (PVLG) (Morton and Benny, 1990; Koske and Tessier, 1983). The spores were then classified to the genus level by observing the colour, size, wall structure and hyphal attachment (Morton and Benny, 1990) and if possible, the species by using a microscope (CX 41 RF, Olympus Corporation, Philippines) and images were captured with a digital camera (Exwave HAD, Sony, Japan).
2.4 SOIL PH
An amount of 10 ml distilled water was added into a flask containing 10 g of soil. The mixture was stirred well and allowed to stand for 10 minutes. The mixture was then filtered with a filter paper to separate the soil from the water. The pH meter (Mettler Toledo AG) was then placed into the solution to measure the pH level (Conklin, 2005). Before measuring the pH, the ion-sensitive electrode was calibrated using a standard buffer solution of pH 7.
2.5 ORGANIC MATTER CONTENT
A small amount of soil (5 g) was oven-dried at 110°C for 2 hours and then left to cool in a dessicator before weighing. The soil was later placed inside a furnace at 500°C for 3 hours and then left to cool overnight. The incinerated soil was again placed in the desiccator before it was weighed (Wilde et al., 1972). The estimation of OM in the soil sample was obtained by using the formula below:

2.6 SOIL MOISTURE
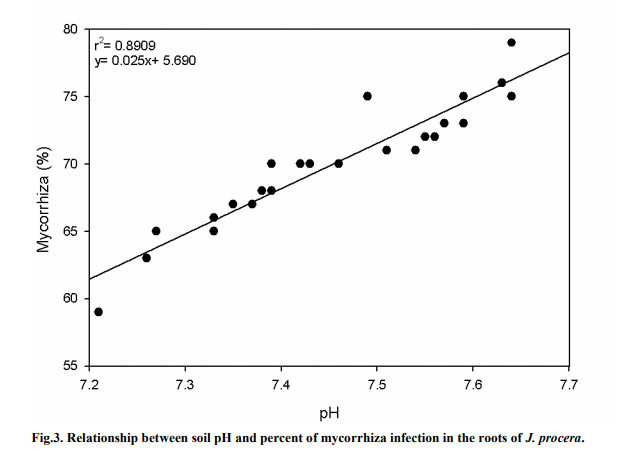
The moisture content was determined by oven drying 100 g of soil at 105°C for 24 hours. Then, the soil was weighed and placed back into the oven to be dried again. This method was repeated until there was no change in weight loss (Yousef, 1999; Conklin, 2005). The estimation of water content was obtained by using the formula below:
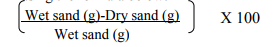
2.7 STATISTICAL ANALYSIS
The linear relationship between soil pH, soil OM and soil moisture with the percent of AMF infection was subjected to Pearson‘s Correlation Coefficient (r2 value). The Correlation Coefficient provides information on the strength of linear relationship. Values closer to 1 indicate a strong linear relationship between combinations. The relationship between soil pH and roots with the percent of AMF infection were determined by using regression analysis. All analyses were performed using Statistix® Version 7.0 (Analytical Software, Tallahassee, Florida).
3. RESULT
3.1 MYCORRHIZAL INFECTION IN J. procera
The stained roots of J. procera were observed under the microscope and found to be heavily infected with AMF about (59-79 %) a lot of endomycorrhizae. A number of endomycorrhizal structures were observed, including hyphal coils that ramify along the root cortex which produces H branches, hyphae with characteristic branching patterns and thin walled intracellular oil-filled vesicles. Thick hyphae were also observed, penetrating the intra- and intercellular region of the cortical tissue. The morphology of the AMF found in the roots of J. procera were of the typical Paris type. It was characterized by the presence of intra- and intercellular hyphal branches. Vesicles were present terminally at the tip of the intra cellular hyphal. Arbuscules were also present in the cortical cells (Figure 1a, 2a and 3a).
3.2 AMF SPORES
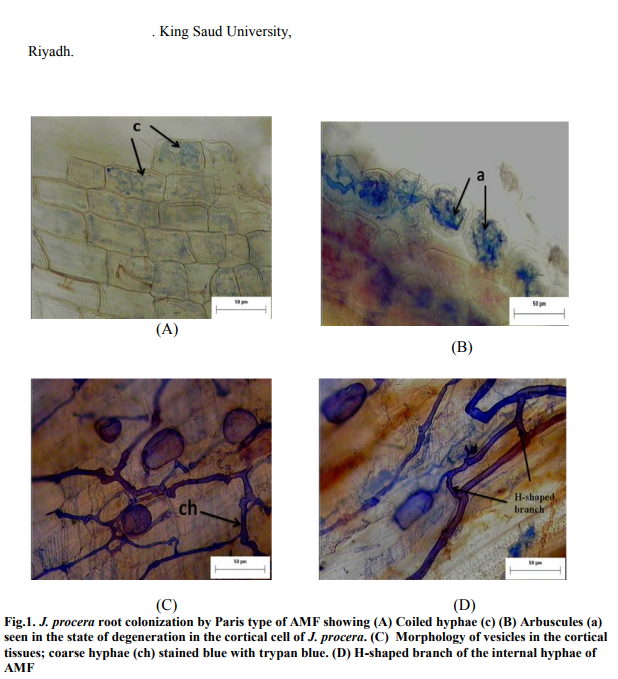
The spores found in the soil sample belonged to the family of Glomaceae, Gigasporaceae and Acaulosporaceae. The spores were identified as Glomus sp., Gigaspora sp., and Acaulospora sp (Figure 2a, 2b, and 2c). Glomus was observed having three layered wall which blends with the wall of the subtending hyphae. Gigaspora was characterized by the appearance of the bulbous attachment of the hyphae an the spore. Acaulospora was identified by the presence of sporiferous saccule.
3.3 SOIL PH
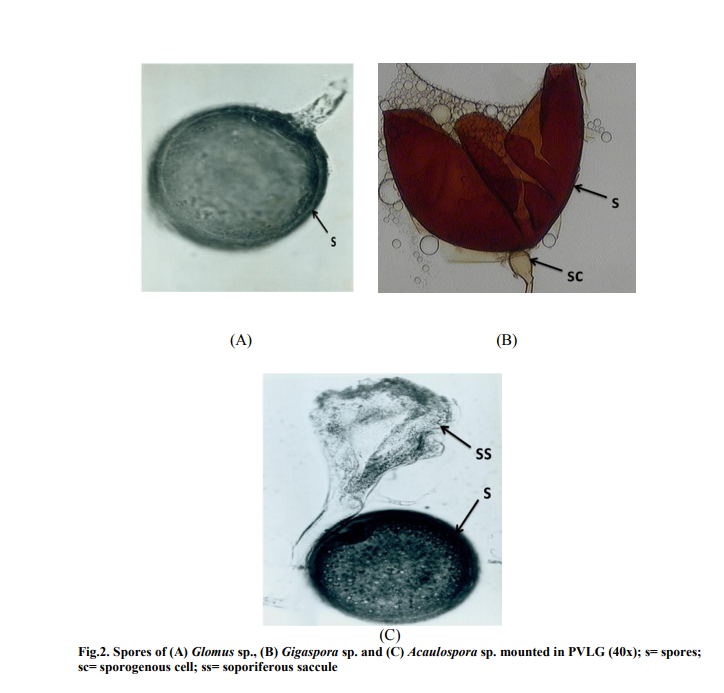
Figure 3 shows a significant relationship between soil pH and the percent of AMF infection on the roots of J. procera (P < 0.05). An r2 value of 0.8909 was recorded, thus showing a strong correlation between the pH value and the AMF infection rate. The pH values in the soil samples were found to be slightly alkaline ranging from around 7.2 to 7.7. A slight increase in soil pH value showed that the AMF infection rate increased considerably. At pH 7.64, around 79% of AMF was found in the root hairs, while at pH 7.21, AMF infection was only at 59%.
3.4ORGANIC MATTER
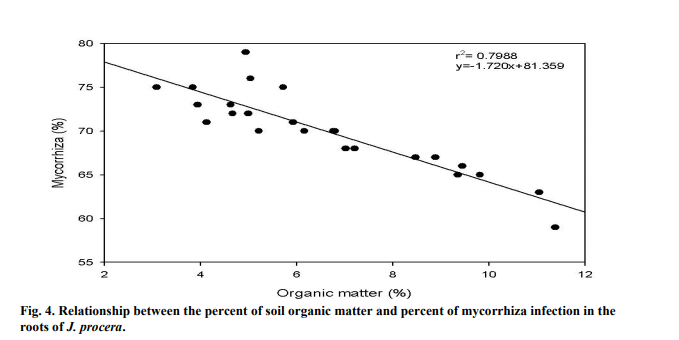
The percent of AMF infection on the roots of J. procera were significantly correlated to the percent of soil OM (P < 0.05). As the OM in the soil increased, the percent of mycorrhiza decreased (Figure 4). The OM in the soil was relatively low, recording between 3.1-11.4% of the total soil sample. The r2 value was recorded at 0.7988, showing that the relations between the two variables are strong.
3.5 SOIL MOISTURE
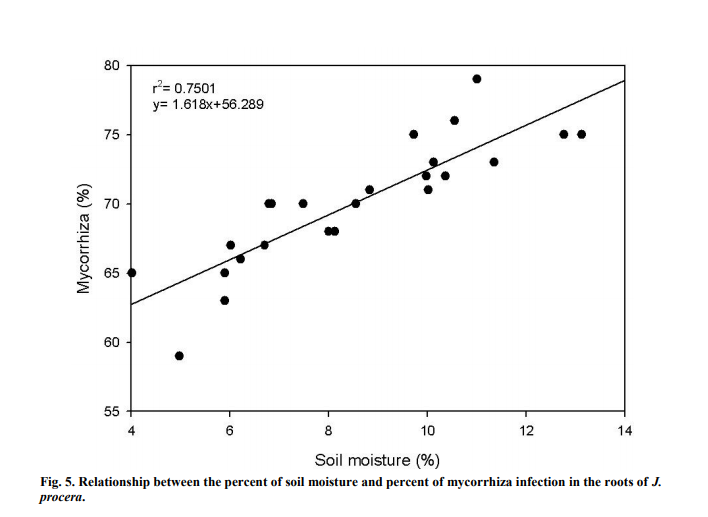
Soil moisture forms a strong and positive relation (Figure 5) with the percent of mycorrhiza infection (r2 =0.7501). Soil moisture significantly influenced the percent of mycorrhiza infection (P<0.05) in the soil samples. Mycorrhiza infection gradually increased as the moisture level in the soil built up. The highest soil moisture was recorded at around 13.1% and the least was at 4.0%.
4. DISCUSSION
4.1MYCORRHIZAL INFECTION IN J. procera
The mycorrhizas in this study were found to grow and developed well in the root samples of J. procera. The association between arbuscular mycorrhizae and their host occurred in many species (e.g. Octomeles sumatrana and Anthocephalus chinensis) living in different habitats (Chubo et al., 2009). Under harsh conditions such as the deserts, arbuscularmycorrhizal fungi (AMF) association with plants is a common phenomenon (Stutz et al., 2000). About 95% of 59 plant species in the hot and arid ecosystem in southwest China were associated with AMF (Li and Zhao, 2005). In addition, Uhlmann et al. (2006) stated that soil and roots samples collected from three arid sites in southern Namibia contained AMF, particularly G. aggregatum. Ephemerals plants living as desert communities, used for grazing and traditional medicine in Junggar Basin, northwest China were also found to be colonized by AMF and formed typical arbuscules or vesicles structures (Shi et al., 2007).
4.2 AMF SPORES
Glomus is classified under Glomales order, while both Gigaspora and Acaulospora are classified under the order of Diversisporales (Walker and Schüßler, 2004). Glomus is characterized by a three-layered wall that blends with the wall of subtending hyphae (Abbas et al., 2006). Gigaspora lacks intra-radical vesicles and the presence of cells does not vary (de Souza et al., 2005). Gigaspora was characterized by the formation of bulbous structure of the subtending hyphae. Spores from the genus Acaulospora was identified by observing the spores that have separated from the sporiferous saccule as it appears to be stalkless (Abbas et al., 2006).
4.3 SOIL PH
Infection of mycorrhiza in this study was recorded from 59 to 79% which corresponds with an increase in soil pH. Soil pH is one of the criteria that affect the growth and colonization of AMF (Kapoor et al., 2002; Abler, 2004; de Boulois et al., 2008). However, AMF response to soil pH is very much influenced by the type of fungal species. Fungal species can either readily form AMF in soils with low or higher pH (Entry et al., 2002). Kumar et al. (2010) found that AM sporulation and the percent of root colonization increased at a higher pH value. It was reported that the number of AM spores and root infection was at its highest at pH 7.2- 7.4 (Selvaraj et al., 2001), while the optimal pH value for mycorrhizal development was reported to vary between pH 6-7 (Sankaranarayanan and Sundarababu, 2001).
4.4 ORGANIC MATTER
In this study, the soil samples contained low amount of OM which is consistent with previous findings. In Saudi Arabia, low amount of OM in the soil was reported by Hashem and Parvez (1994). AMF benefits from the soil surrounding the tree rhizospheres as it contains humified organic matter which mixes with soil minerals (Neelam et al., 2010). In the Malakand division of Pakistan, a study found that the rate of AMF infection in the roots of wheat and maize crops were correlated to the composition of organic matter. The study found that soils with poor organic matter composition have greater infections with AM (Sharif et al., 2010).
4.5 SOIL MOISTURE
This study found that mycorrhiza infection in the root samples was higher with higher soil moisture. Lack of water is one of the most common problems inhibiting plant growth (Aroca et al., 2008). However, reports have shown that the same physiological processes can be altered through AM symbiosis in addition to helping plants withstand water deficiency (Augé, 2001; Augé, 2004; Aroca et al., 2008). Under stressful situations such as drought, AMF help plants to grow much better through AMF symbiosis (Porcel et al., 2003). The plant will provide carbon molecules to the AMF and in return the plants will acquire nutrients, particularly phosphorus and water from AMF and this subsequently enhance plant growth (Gosling et al., 2006). According to Kumar et al. (2010), the density of mycorrhiza infection is dependent upon soil moisture. AMF can cause an increase in the absorption of water and nutrients because of the extensive network of hyphae as it increases soil contact with the roots (Miransari et al., 2009). In arid and semiarid ecosystems, roots of plants usually have a large amount of AM fungal root colonization. This shows that under limited supply of water, AMF symbiosis is of great importance for plant growth (Tchabi et al., 2008). Spores germination and development of fungal propagules needs moisture during the early stages of their development despite the fact that AMF helps plant to be more tolerable towards drought (Al-Karaki et al., 2004). Augé (2001) stated that AMF can only provide help in enhancing the tolerability of plants towards drought once the association between AMF and plants has been established.
5. CONCLUSION
In this study, soil physical properties were examined and related to the percent of AMF in the roots of J. procera. AMF is known to benefit plants by improving the conditions of the soil. AMF in the roots of J. procera was significantly affected by soil pH, soil moisture and organic matter. AMF percent in the roots of J. procera increased with an increase of soil pH and moisture level. On the contrary, AMF colonization was high when organic matters were low. Changes in soil pH, moisture and organic matters can influence the abundance of AMF and considerably affect their development.
ACKNOWLEDGEMENT
We would like to acknowledge King Fahd Medical Research Center (Jeddah, Saudi Arabia) and Universiti Sains Malaysia (Penang, Malaysia) for providing the apparatus and materials required in this study. This study was financially supported by King Abdul Aziz University (Jeddah, Saudi Arabia).
References:
1. Abbas Y, Ducousso M, Abourouh M, Azcón R and Duponnois R (2006). Diversity of arbuscular mycorrhizal fungi in Tetraclinis articulata (Vahl) Masters woodlands in Morocco. Ann. For. Sci., 63: 285– 291.
2. Abler RAB (2004). Trace metal effects on ectomycorrhizal growth, diversity, and colonization of host seedlings. Ph.D dissertation, Blackburg, Virginia.
3. Aroca R, Vernieri P and Ruiz-Lozano JM (2008). Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J. Exp. Bot., 59: 2029-2041.
4. Al-Gamdi MAA (2006). The possibility of ameliorating the regeneration of Juniper trees (Juniperus procera Hochst. ex Endl.) in the natural forests of Saudi Arabia. Msc. Dissertation. University of King Saud, Riyadh.
5. Al-Karaki G, McMichael B and Zak J (2004). Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza. 14: 263-269.
6. Augé RM (2001). Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza., 11: 3– 42.
7. Augé RM (2004). Arbuscular mycorrhizae and soil/plant water relations. Can. J. Soil Sci., 84: 373–381.
8. Brachmann A and Parniske M (2006). The most important symbiosis on earth. J. Soil Biol., 4: 239.
9. Bray EA (1997). Plant responses to water deficit. Trends Plant Sci., 2: 48-54.
10. Brundrett M, Bougher N, Dell B, Grove T and Malajczuk N (1996). Chapter 4: Examining mycorrhizal associations. In: Working with Mycorrhiza in Forestry and Agriculture. Australian Centre for International A Bavaresco gricultural Research, Canberra, Australia. P. 173-215.
11. Conklin AR (2005). Introduction to Soil Chemistry. Analysis and Instrumentation, 3 rd edition, John Wiley and Sons, Inc., Publication, Hobokon, New Jersey.
12. de Boulois HD, Joner EJ, Leyval C, Jakobsen I, Chen BD, Roos P, Thiry Y, Rufyikiri G, Delvaux B and Declerck S (2008). Impact of arbuscular mycorrhizal fungi on uranium accumulation by plants. J. Environ. Radioactiv., 99: 775-784.
13. de Souza FA, Dalpé Y, Declerck S, de Ia Providencia IE and Séjalon-Delmas N (2005). Life history strategies in Gigasporaceae: Insiight from Monoxenic culuture. Chapter 5. In: Declerk S, Strullu D-G, Fortin JA (editors.), In vitro culture of mycorrhizas. Springer-Verlage Berlin Heidelberg.
14. Entry JA, Rygiewicz PT, Watrud LS and Donnelly PK (2002). Influence of adverse soil conditions on the formation and function of arbuscular mycorrhizas. Adv. Environ. Res., 7: 123-138.
15. Gosling P, Hodge A, Goodlass G and Bending GD (2006). Arbuscular mycorrhizal fungi and organic farming. Agriculture Ecosyst. Environ., 113: 17–35.
16. Hajar AS, Faragalla AA, Al-Ghamdi KM (1991). Impact of biological stress on Juniperus excelsa M. Bieb. in south-western Saudi Arabia: insect stress. J. Arid Environ., 21: 327-330.
17. Hashem AR, Parvez S (1994). Mycoflora of Aluminium Rich of Hail Region, Saudi Arabia. Arab Gulf J. Sci. Res., 12: 34-350.
18. Kapoor R, Giri B and Mukerji KG (2002). Soil factors in relation to distribution and occurrence of versicular arbuscular mycorrhiza. In: Mukerji KG, Manoharachary C, Chamola BP (editors.), Techniques in Mycorrhizal Studies. Kluwer Academic Publisher, Dordrecht, The Netherlands.
19. Koske RE and Tessier BA (1983). A convenient permanent slide mounting medium. Mycological society of America newsletter, 3: 59.
20. Kumar A, Mangla C, Aggarwal A and Parkash V (2010). Arbuscular mycorrhizal fungal dynamics in the rhizospheric soil of five medicinal plant species. Middle-East J. Sc. Res., 6: 281-288.
21. Li T and Zhao Z (2005). Arbuscular mycorrhizas in a hot and arid ecosystem in southwest China. Appl. Soil Ecol., 29:135– 141.
22. Miransari M, Bahrami HA, Rejali F and Malakouti MJ (2008). Using arbuscular mycorrhiza to reduce the stressful effects of soil compaction on wheat (Triticum aestivum L.) growth. Soil Biol. Biochem., 40: 1197–1206.
23. Miransari M, Bahrami HA, Rejali F and Malakouti MJ (2009). Effects of soil compaction and arbuscular mycorrhiza on corn (Zea mays L.) nutrient uptake. Soil Till. Res., 103: 282–290.
24. Morton JB and Benny GL (1990). Revised classification of arbuscular mycorrhizal fungi (Zygomycetes): a new order, Glomales, two new suborders, Glomineae and Gigasporineae, and two new families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon., 37: 471-491.
25. Neelam V, Tarafdar JC and Srivastava KK (2010). Periodic changes in Prosopis cineraria associated AM population at different soil depth and its relationship with organic carbon and soil moisture. Afr. J. Microbiol. Res., 4: 115-121.
26. Negash L (2002). Successful vegetative propagation techniques for the threatened African pencil cedar (Juniperus procera Hoechst. ex Endl.). For. Ecol. Manage., 161: 53-64.
27. Porcel R, Barea JM. and Ruiz-Lozano JM (2003). Antioxidant activities in mycorrhizal soybean plants under drought stress and their possible relationship to the process of nodule senescence. New Phytol., 157: 135– 143.
28. Sankaranarayanan C and Sundarababu R (2001). Influence of moisture and pH on the efficiency of vesicular arbuscular mycorrhiza, Glomus mosseae against Meloidogyne incognita on black gram (Vigna mungo L.). J. Biol. Control., 15: 69- 72.
29. Sharif NM, Rubina K, Burni T (2010). Occurrence and distribution of arbuscular mycorrhiza in wheat and maize crops grown in northern areas of North West Frontier Province. Pak. J. Bot., 42: 1301-1312.
30. Shi ZY, Zhang LY, Li XL, Fen G, Tian CY and Christie P (2007). Diversity of arbuscular mycorrhizal fungi associated with desert ephemerals in plant communities of Junggar Basin, northwest China. Appl. Soil Ecol., 35: 10-20
31. Stutz JC, Copeman R, Martin CA and Morton JB (2000). Patterns of species composition and distribution of arbuscular mycorrhizal fungi in arid regions of southwestern North America and Namibia, Africa. Can. J. Bot., 78: 237-245. 3
2. Tchabi A, Coyne D, Hountondji F, Lawouin L, Wiemken A and Oehl F (2008). Arbuscular mycorrhizal fungal communities in sub-Saharan Savannas of Benin, West Africa, as affected by agricultural land use intensity and ecological zone. Mycorrhiza., 18: 181- 195.
33. Uhlmann E, Görke C, Petersen A and Oberwinkler F (2006). Arbuscular mycorrhizae from arid parts of Namibia. J. Arid Environ., 64: 221–237.
34. Walker C and Schüßler A (2004). Nomenclatural clarifications and new taxa in Glomeromy- cota. Mycol. Res., 108: 981- 982.
35. Wilde SA, Voigt GK and Iyer, JG (1972). Part 1: Analysis of physical properties of soils, 4 th edition, In: Chesters G (editors.), Soil and Plant Analysis for Tree Culture. Madison. WI. P. 6-34.
36. Yousef AF (1999). Analysis methods and devices for soil and water. In Arabic:
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License