IJCRR - 3(10), October, 2011
Pages: 33-37
Print Article
Download XML Download PDF
ANTI-MICROBIAL EFFECT OF AZADIRACHTA INDICA AND OCIMUM GRATISSIMUM AGAINST ASPERGILLUS NIGER CAUSING STORAGE ROT OF GARCINIA KOLA
Author: Amadioha, A. C., Obi, Uchenna O.
Category: General Sciences
Abstract:Three fungi Aspergillus niger, Penicillium expansum and P. digitatum were isolated from seeds of bitter
kola (Garcinia kola) obtained from market stalls in Umuahia, Abia State, Nigeria but A. niger was found
pathogenic during pathogenicity test. The pathogenic organism was subjected to cold water leaf extracts
of A. indica (Neem) and O. gratissimum (Sweet Basil) which gave a growth inhibition of 85.35 % and
66.74 % respectively after five days of incubation. The inhibitory effects of the extracts against the
pathogen were more with A. indica (Neem) than O. gratissimum and this increased with the incubation
period. Cold water extracts of these plants could be exploited as pesticides of plant origin in the control of
post-harvest microbial deterioration of seeds of G. kola incited by A. niger.
Full Text:
INTRODUCTION
Garcinia kola commonly referred to as bitter kola due to its bitter taste is highly medicinal (Uko et. al., 2001) and usually found in the tropical rain forest region of West and Central Africa (Gill, 1998). Seeds of G. kola are highly nutritious (Adeyaye et. al., 2007) and several medicinal and anti-microbial attributes like treatment of cough and hepatitis, snake repellant, and chest medicine have been assigned to the seeds of G. kola ( Iwu, 1993). Post-harvest microbial deterioration of G. kola seeds has been observed during storage and this has reduced both the nutritional and market value of the seeds. Extending the shelf-life of G. kola seeds during storage will ensure availability of the seeds and continuous production (Korie, 1996). Depletion of important forest trees like G. kola in rainforest regions as well as the medicinal values of G. kola seeds which have increased dramatically in the last decade (Smith, et. al., 1996) makes it imperative for an increased search for methods of preserving seeds of G. kola. Available literature indicates that not much work has been done on the microbial deterioration of G. kola seeds and their control in storage. Studies on anti-microbial activity of plant extracts have shown the significance of natural chemicals as possible source of non-phytotoxic, systemic and easily biodegradable alternative to synthetic pesticides which are not only hazardous to both the farmer and the environment but scarce and expensive when available (Amadioha, 1998, 2003; Olojede et. al., 1993). Extracts of A. indica (Neem) and O. gratissimum have been reported to be very effective in the control of storage disease of some other agricultural products (Amadioha and Obi, 1999). The effectiveness of leaf extracts ofthese plants in the control of storage rot disease of seeds of G. kola incited by A. niger is presented in this paper.
MATERIALS AND METHODS
Source of Garcinia kola: Matured healthy (uninfected) and infected seeds of G. kola were obtained from open market stalls at Oboro, Umudike and Ndume in Umuahia, Abia State, Nigeria. The infected samples were collected in sterile polyethylene bags and taken to the laboratory for isolation. Culture Medium: Potato Dextrose Agar (PDA) was used in the isolation of the fungi associated with the rot of G. kola seeds. Thirty-nine grammes of PDA was added to one litre of distilled water containing 1000mg of chloramphenicol, thoroughly mixed and dispensed into 500ml conical flasks and then plugged with cotton wool and capped with aluminum foil before sterilization for 15 minutes in an autoclave set at 121oC (105kg/cm2 ). The sterilized medium (20ml) was dispensed into sterile disposable Petri dishes (9 cm diameter). Isolation and Identification of Isolates: Infected G. kola seeds were washed in running tap water and distilled water before surface sterilizing with 70% ethanol for 10 seconds to prevent any surface contaminant that may interfere with the isolation and identification of rot causing organisms. Infected tissues (5cm) were removed and plated on the chloramphenicol amended culture medium (PDA) in Petri dishes and incubated for 5 days at room temperature (27oC). Three isolates were identified, A. niger, P. expansum and P. digitatum based on their morphological characteristics and reference to Rossman et. al. (1997). Pathogenicity Test: The method described by Amadioha (1998) was used. Healthy (uninfected) G. kola seeds were disinfected with70% ethanol and rinsed with distilled water With the aid of a cork borer (4 mm diameter), a 4 mm diameter disc was removed and a 4 mm diameter disc of the 5- day old culture of the isolates were each used to plug each hole. The disc removed from the healthy G. kola seed was replaced after 1 mm had been cut off to compensate the thickness of the isolate and then sealed with Vaseline. Each inoculated seed was placed in a micro-humidity chamber (a small polyethylene bag containing cotton wool soaked with distilled water) and incubated for 5 days. Following the development of rot symptoms, reisolation was carried out to confirm that the isolates were the same as the original isolates introduced. The organism that caused rot and found to possess the same characteristics features with the original isolate (A. niger) was confirmed as pathogen whereas the Penicillium species that did not cause any rot symptom during pathogenicity test were regarded as nonpathogens or saprophytes and discarded. Effect of extracts of Azadirachta indica and Ocimum gratissimum on the radial growth of Aspergillus niger : Fresh leaves of A. indica (Neem) and O. gratissimum were washed in running tap water and sterile distilled water, airdried at 27oC, weighed (100g) and ground in a sterile mortar with a piston. The paste was put in 250ml beaker and 100ml of distilled water was added, stirred vigorously and allowed to stand for 1 hr and then filtered through four folds of sterile cheese cloth to obtain a cold water extract of each of the test plants. The effect of the extracts on the radial growth of A. niger was determined using the poisoned food technique described by Amadioha (2002). The extract-PDA medium was prepared by spreading 0.5ml of each extract separately on the surface of the solidified PDA contained in Petri-dishes to form a thin film. The control was 0.5ml of sterile distilled water. A disc (5mm diameter) of 5 day old culture of A. niger was cut from the growing end of its pure culture and placed in the centre of a Petri-dish with three replicates of each treatment. The treated plates and control were then incubated at 27oC and radial growth in each treated plate and control experiment was measured after 4 days when the fungal growth in the control experiment had reached the periphery of the Petri-dishes. The experiments were repeated four times and mean values obtained. The percentage growth inhibition was calculated using the formula adopted by Amadioha (2003):

RESULTS AND DISCUSION
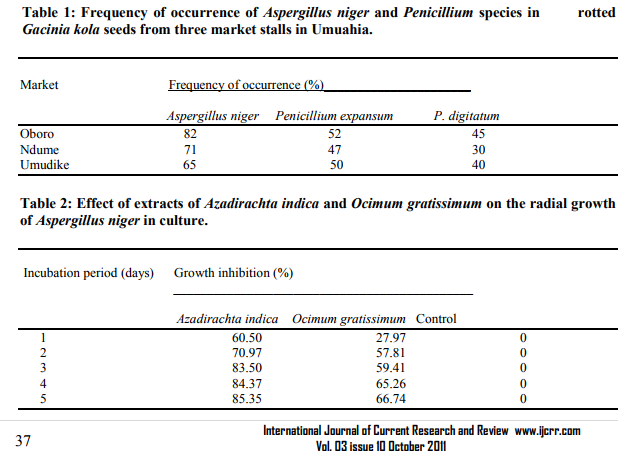
Isolation and identification of pathogenic organism: Three fungi were isolated from dead remains of G. kola, A. niger, P. expansum and P. digitatum but A. niger was pathogenic during the pathogenicity test. The frequency of association is shown in Table 1. A. niger was used as test organism throughout the course of this experiment based on its frequency of occurrence and level of pahogenicity. Several pathogenic organisms have been associated with the postharvest microbial deterioration of stored agricultural products (Amadioha and Adisa, 1999; Markson et. al., 2010). Booth (1974) and Coursey (1967) attributed attack by microorganisms as the most serious cause of post-harvest loss of stored products. In the present study, A. niger caused appreciable rot of G. kola seeds in storage whereas the Penicillium spp were found to be saprophytic or nonpathogenic. This is apparently the first report in Nigeria showing A. niger as the most virulent and frequently encountered pathogen inciting rot of G. kola seeds in storage. Effects of Azadirachta indica and Ocimum gratissimum leaf extracts on the radial growth of Aspergillus niger in vitro: Potential use of extracts of plant origin in plant disease control has been emphasized (Amadioha, 2000) but very little or no work has been done on the use of plant products against storage rot of G. kola seeds caused by A. niger. Results of the effects of A. indica (Neem) and O. gratissimum leaf extracts on A. niger showed that the plant extracts significantly inhibited the radial growth of test fungus in vitro when compared with the control experiment, suggesting the presence of antifungal substances in the tissues of the test plants. A. indica was more effective than O. gratissium in reducing the radial growth of the pathogen in culture. The differences in toxicity could be attributed to the solubility of the active principles/compounds of the test plants in the extracting solvent and or, the presence of inhibitors to the active principle (Amadioha, 2001), with higher solubility of the active compounds of A. indica than O. gratissium. The differences in active principles/compounds of the test plants and their solubility in the extracting solvents could be influenced by the age of the plant and the extracting solvent (Qasem and Abu-Blan, 1996). A. indica, has been reported to be effective as insecticide (Emosairue and Ukeh, 1990), bird repellent (Mason and Mathew, 1996) and as fungicide both in the field and storage (Amadioha, 2001, 2002; Amadioha and Obi. 1998). The current investigation showed that the percentage growth inhibition of the pathogen in culture increased with period of incubation (Table 2), indicating the presence and persistence of the anti-fungal activity of the extracts of the test plants which were retained against the pathogen for the whole period of incubation. The test plants are common medicinal plants in Nigeria that could be exploited as extract of plant origin for the control of storage rot disease of G. kola seeds incited by A. niger.
CONCLUSION
A survey of three market stalls in Umuahia Abia State, Nigeria showed that A. niger was a major pathogenic organism causing storage rot of G. kola seeds. In vitro investigations revealed that cold water crude leaf extracts of A. indica (Neem) and O. gratissimum inhibited the radial growth of A. niger in culture suggesting the presence of anti-microbial substances in the tissues of these plants. The extracts of A. indica (Neem) and O. gratissimum could be exploited as pesticides of plant origin in the control of post-harvest microbial deterioration of G. kola seeds caused by A. niger.
References:
REFERENCES
1. Adeyaye, C.J; Asaolu, S.S; Aluko, A.O. (2007) Amino acid composition of two masticatry nut Cola acuminate and Garcinia kola and Anacardium occidentale ( Snack nut). International Journal of Food Science and Nutrition 58, 241-249.
2. Amadioha, A. C. (1998) Control of postharvest tuber rot of potato incited by Rhizoctonia bataticola. Archives of Phytopathology and Plant Protection 31, 225-231.
3. Amadioha, A.C. (2000) Controlling Rice blast in vitro and in vivo with extracts of Azadirachta indica. Crop Protection 19, 287 – 290.
4. Amadioha, A.C. (2001) Fungicidal activity of some plant extracts against Rhizoctonia solani in cowpea. Archives of Phytopathology and Plant Protection 33, 509 –517.
5. Amadioha, A.C. (2002) Fungitoxic effects of extracts of Azadirachta indica against Cochliobolus miyabeanus causing brown spot disease of rice. Archives of Phytopathology and Plant Protection 35, 37- 42.
6. Amadioha, A. C. (2003) Evaluation of some plant leaf extracts against Collectotrichum lindemuthianmum in cowpea. Acta Phytopathologica 38, 259-265.
7. Amadioha, A. C. and Adisa, V. A. (1999) Postharvest microbial deterioration of potato tubers (Solanum tuberosum L.) in Nigeria. Journal of Tropical Root Crops 3(2), 40- 43.
8. Amadioha , A. C. and Obi, V. I. (1998) Fungitoxic activity of Azadirachta indica and Xylopia aethiopica on Colletotrichum lindemuthianum. Journal of Herbs, Spices and Medicinal Plants. 6, 33-40.
9. Amadioha, A. C. and Obi, V. I. (1999) Control of anthracnose disease of cowpea by Cymbopogon citratus and Ocimum gratissimum. Acta Phytopathologica et Entomotogica 34, 85 –89.
10. Booth, R.H. (1974) Post-harvest deterioration of tropical root crops; losses and their control. Tropical Science 16, 49-63
11. Coursey, D.G. (1967) Yam storage. A review of yam storage practices and information on storage losses. Journal of Stored Products Research 2, 209-243.
12. Emosairue, S.O. and Ukeh, D.A. (1996) Field trial of neem products for the control of okra fleabeetles (Podasgrica spp) in Southern Nigeria. African Journal of Plant Protection 6, 27-33.
13. Gill, L.S. (1998) Taxanomy of flowering plants. New York Science Journal 3(3), 156 – 160.
14. Iwu, M.M. (1993) Hand book of African medicinal plants. CRC Press Boca Raton, F.L. 223-224.
15. Korie, C.N. (1996) Effects of different packaging materials on the shelf life of kola. B. Agric. Tech (crop protection) project report. Federal University of Technology, Owerri. 62pp.
16. Markson, A.A., Amadioha, A.C., Wokocha, R.C., Omosun, G. and Madunagu, B.E. (2010) Biochemical alteration of yam tuber tissues incited by Botryodiplodia theobromae Pat. International Journal of Current Research 4, 94-97.
17. Mason, J.R. and Mathew, D.N. (1996) Evaluation of neem as a bird repellant chemical. International Journal of Pest Management 42, 47-49.
18. Olojede, F., Engelhardt, G., Wallnofor, P.R. and Adedoke, G.O. (1993) Decrease of growth and aflotoxin production byAspergillus parasiticus. World Journal of Microbiology and Biotechnology 2, 605-606
19. Qasem, J.R. and Abu-Blan, H.A. (1996) Fungicidal activity of some common weed extracts against different plant pathogenic fungi. Journal of Phytopathology 144, 157- 161.
20. Rossman, A.Y., Palm, M.E. and Spielman, L.J. (1997) A literature guide for the identification of plant pathogenic fungi. APS press, Minnesota, USA.
21. Smith, G.M.., Clegg, C., Keem, N. and Grivetti, T. (1996) Mineral values of isolated plant foods common to Southern Burkina Faso, Niamey and Nigeria, West Africa. International Journal of Food Science and Nutrition 47, 41-53.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License