IJCRR - 3(12), December, 2011
Pages: 17-22
Print Article
Download XML Download PDF
PHYTOCHEMICAL AND ANTIMICROBIAL STUDIES OF THE LEAF EXTRACTS OF BUCHOLZIA CORIACEAE
Author: P. O. Osadebe, G. A. Awemu, K.M.Ezealisiji, M.O.Agbo
Category: Healthcare
Abstract:Bucholzia coriaceae has been reported in folk medicine to have many medicinal properties
including anthelmentic,antimicrobial and antifungal activity. Phytochemical screening of the
aqueous and methanol extracts of Bucholzia coriaceae leaves revealed the presence of tannins,
reducing sugars, saponins, terpenoids, flavonoids, and cardiac glycosides. The minimum
inhibitory concentration (MIC) of the methanol extract was between 0.5 \? 7.0 mg/ml while the
minimum bactericidal concentration (MBC) ranged from 2.0 \? 9.0 mg/ml. The methanol and
aqueous extracts exhibited antifungal activity against Candida albicans and Aspergillus niger
with zones of inhibition of 11.5 mm and 3.6 mm for Candida albicans ; 9.0 mm and 2.0 mm
for Aspergillus niger. The aqueous extract exhibited less antimicrobial effect than the
methanol extract.
Keywords: Bucholzia coriaceae, antimicrobial, zone of inhibition
Full Text:
INTRODUCTION
Microorganisms are increasingly developing resistance against commonly used antimicrobial agents and the use of plants in the treatment of diseases is becoming widely accepted (Peni et al, 2010). Phytochemicals from plants may serve as new sources of antimicrobial agents possibly with novel mechanisms of action (Jigna et al, 2005) and contrary to synthetic drugs, antimicrobial agents of plant origin are not associated with the myriad of side effects associated with synthetic drugs and have enormous potentials to heal many infectious diseases (Iwu et al, 1999; Umerie and Emelugo, 2007).
Bucholzia coriaceae is a forest tree with large, glossy, leathery leaves and conspicuous cream white flowers in racemates at the end of the branches. The plant is easily recognized by the compound pinnate leaves and the long narrow angular fruits containing large, usually aligned seeds (Mbata et. al, 2009). The leaves and stem bark in various formulations exhibit antihelminthic, and cytotoxic effects (Ajaiyeoba et. al, 2001; Ajaiyeoba et. al, 2003; Nweze and Asuzu, 2006; Ezekiel and Onyeoziri, 2009). In Ghana, the fresh bark of the plant is used the treatment of for ear ache (Irvine, 1961).
Despite the wide spread applications of extracts of the plant parts of Bucholzia coriacea in African traditional system of medicine, scientific investigations are concentrated mostly on the seed extract. The present study therefore evaluates the antibacterial, antifungal and phytochemical profile of the methanol and aqueous extracts of the leaf of Bucholzia coriaceae.
MATERIALS AND METHODS
Plant Material Fresh leaves of Bucholzia coriacea were harvested in the month of June 2010 from Elele, Rivers State Nigeria.The leaves were identified and authenticated by Pharm. Osuala of the Department of Pharmacognosy, Faculty of Pharmacy, Madonna University, Elele. A voucher specimen of the sample was deposited in the herbarium of the department. The leaves were air dried for 14 days and pulverized.
EXTRACTION OF THE PLANT MATERIAL
Five hundred grams (500 g) of the powdered leaves were separately extracted with 2.0 L methanol and 2.0 L distilled water by cold maceration at room temperature for 24 h. The methanol extract was concentrated in vacuo using a rotary evaporator while the aqueous extract was lyophilized.
QUALITATIVE PHYTOCHEMICAL TESTS
Phytochemical analyses of the extracts were carried out using the procedure outlined by Trease and Evans (1983).
MICROORGANISMS
Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Klebsiella pneumonia, Salmonella typhii, Bacillus subtilis, Pseudomonas aeruginosa, Candida albicans and Aspergillus niger were used in this study. These microorganisms were obtained from the Federal Medical Center (FMC), Owerri, and Imo State, Nigeria as clinical isolates and maintained in nutrient broth media at 37oC in the Department of Pharmaceutics and Pharmaceutical Microbiology, Madonna University, Elele.
ANTIBACTERIAL ACTIVITY
The antibacterial activities of the extracts were determined using with the agar well diffusion method (Irobi et al, 1994; Igbinosa et al, 2009). The bacterial isolates were first grown in nutrient broth for 18 h before use and standardized to 0.5 McFarland standards (106 cfu/ml). Two hundred microlitres of the standardized cell suspension was spread on a Mueller – Hinton agar (Oxoid). Wells were then bored into the agar using a sterile 6 mm diameter cork borer. Approximately 100 µL of the crude extract at 10 mg/ml were introduced into the wells, allowed to stand at room temperature for about 2 hours and then incubated at 37oC. The plates were observed for zones of inhibition after 24 hours. The effects were compared with those of Ciprofloxacin at a concentration of 1 mg/ml.
ANTI FUNGAL ACTIVITY
The fungal isolates were allowed to grow on a sabourand dextrose agar (SDA) (Oxoid) at 25oC until they sporulated. The fungal spores were harvested after sporulation by pouring a mixture of sterile glycerol and distilled water to the surface of the plate and later the spores were scraped with a sterile glass rod.
MINIMUM INHIBITORY CONCENTRATION (MIC)
The MIC was determined for the microorganisms that were sensitivity to the test extracts. The broth dilution method was used for MIC determination according to the method of Vollekova et al (2001). The prepared broth was poured in ten test tubes and inoculated with 2 ml of the sensitive microorganisms. Several dilutions of the extract and standard were prepared and 0.1 ml of each was transferred in each test tube and labeled. The test tubes were inoculated for 24 hrs at 37oC and the least concentration in which no growth of the microorganisms (absence of turbidity) occurred was noted as the minimum inhibitory concentration.
MINIMUM BACTERICIDAL CONCENTRATION (MBC)
The method of Spencer and Spencer (2004) was used for the MBC determination. Samples were taken from the plates with no visible growth in the MIC assay and subcultured on freshly prepared nutrient agar plates and SDA plates, and later incubated at 37oC for 48 h for bacteria and 25oC for 72 h for fungi respectively. The MBC was taken as the concentration of the extract that did not show any growth on a new set of agar plates.
RESULTS AND DISCUSSION
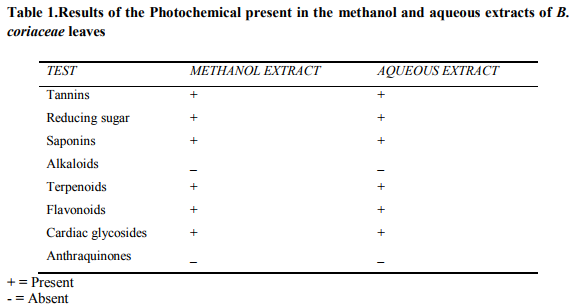
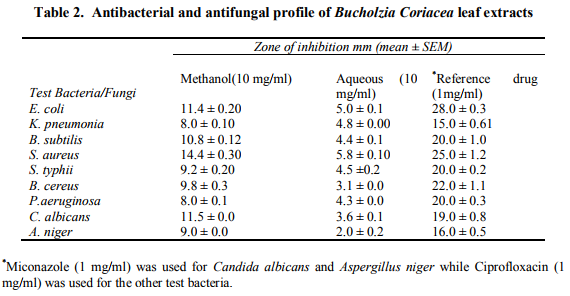
Phytochemical screening of the aqueous and methanol extracts of Bucholzia coriaceae leaves revealed the presence of tannins, reducing sugar, saponins, terpenoids, flavonoids and cardiac glycosides (Table 1). These compounds protect the plants against infections caused by by microorganisms and predation by pests and animals (ElMahmood and Amey, 2007; ElMahmood and Doughari, 2008). A major contribution of higher plants to both traditional and biomedicine healthcare systems is the limitless capability of the plants to produce a large number of phytochemicals of high structural diversity (ElMahmood and Doughari, 2008) and there is a sence of urgency in studying medicinal plants to unveil their pharmaceutical secrets before deforestation takes its toll and reduce the chances of finding novel drugs (Lambert, 2001). Both extracts of Bucholzia coriaceae siginificantly inhibited the growth of :Escherichia coli, Klebsiella pneumonia, Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa and the fungi, Candida albicans and Aspergillus niger (Table 2). The activity of the plant extracts against both Gram positive and Gram negative bacteria is an indication that the extracts have broad spectrum antibiotic effects (Pareekh and Chanda, 2007). Traditionally, the fresh leaves of Bucholzia coriaceae are boiled and the aqueous extract taken orally as anthelmintic, antimicrobial or antifungal agent (Ajaiyeoba et. al, 2003). The methanol extract showed greater antimicrobial activity than the aqueous extract probably due to the presence of soluble phenolic and polyphenolic compounds (Kowalski and Kedzia, 2007).
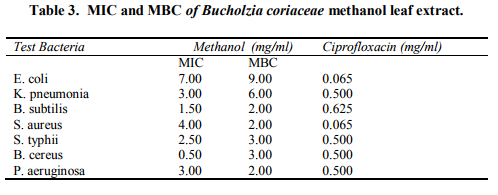
The minimum inhibitory concentration (MIC) of the methanol extract for different organisms ranged between 0.5 mg/ml and 7.0 mg/ml. The MIC of ciprofloxacine ranged between 0.065 and 0.625 mg/ml. The minimum bacterial concentration (MBC) of the extracts for different bacteria ranged between 2.0 - 9.0 mg/ml (Table 3).
The effects of the crude extracts correlate with the reports that microorganisms vary widely in their degree of susceptibility to agents (Emeruwa, 1982). High MIC and MBC values are indicative of low activity (ElMahmood and Doughari, 2008).
In this study, Escherichia coli and Klebsiella pneumonia had higher MIC values, thus suggesting lower susceptibility to the efficacy of the methanol extract and lower values for Bacillus subtilis, Staphylococcus aureus, Salmonella typhii, Bacillus cereus and Pseudomonas aeruginosa suggest high susceptibility to the efficacy of the methanol extract. The results of the study have shown that the aqueous extract of Bucholzia coriacea leaves which is widely used in African traditional system has very weak antibacterial and antifungal activity.
ACKNOWLEDGMENTS
The authors are grateful to Mr. A. O. Ozioko of Bioresources Conservation and Development Programme (BCDP), Nsukka for his numerous assistance in sourcing for the plant materials and authenticating them.
References:
1. Ajaiyeoba, E. O., Onocha, P. A., Olarenwaju, O. T. (2001). In vitro antihelminthic properties of Bucholzia coriaceae and Gynandropsis gynandra extracts. Pharm. Biol. 39(3): 217 – 222.
2. Ajaiyeoba, E. O., Onocha, P. A., Nweze, S. O., Sama, W. (2003). Antimicrobial and cytotoxicity evaluation of Bucholzia coriaceae stem bark. Fitoterapia. 74(7 – 8): 706 – 709.
3. Burkill, H. M. (1985). The useful plants of West Africa. Royal Gardens, Kew, P. 319.
4. ElMahmood, A. M., Amey, J. M. (2007). In vitro antibacterial activity of Parka biglobosa (Jacq) root bark extracts against some microorganisms associated with urinary infections. African Journal of Biotechnology .6(11): 1272 – 1275.
5. ElMahmood, A. M., Doughari, J. H. (2008). Phytochemical screening and antibacterial evaluation of the leaf and root extracts of Cassia alata Linn. African Journal of Pharmacy and Pharmacology .2(7): 124 – 129.
6. Emeruwa, K. C. (1982). Antimicrobial substances from Carica papaya fruit extracts. Journal of Natural Products .45(2): 123 – 127.
7. Ezekiel, O. O., onyeoziri, N. F. (2009). Preliminary studies on the antimicrobial properties of Bucholzia coriaceae (Wonderful kola). African Journal of Biotechnology. 8(3): 472 – 474.
8. Igbinosa O. O., Igbinosa E. O., Aiyegoro O. A. (2009). Antimicrobial activity and phytochemical screening of stem bark extracts from Jatropha curcas (Linn). African Journal of Pharmacy and Pharmacology. 3(2): 058 – 062.
9. Irobi O. N., Moo – Young M, Anderson, W. A., Daramola, S. O. (1994). Antimicrobial activity of the bark of Bridelia ferruginea (Euphorbiaceae). International Journal of Pharmacognosy. 34: 87 – 90.
10. Irvine, F. R. (1961). Woody plants of Ghana with references to their uses. Oxford University Press, London. Pp. 233 – 237.
11. Iwu, M. M., Duncan, A. R., Okunji, C. O. (1999). New antimicrobials of plant origin. In: Janick, J. (Ed). Perspectives of new crops and new uses. ASHS Press, Alexandria, USA. Pp. 457 – 462.
12. Jigna, P., Rathish, N., Sumitra, C. (2005). Preliminary screening of some folklore medicinal plants from Western India for potential antimicrobial activity. Journal of Pharmacology. 39: 408 – 409.
13. Kowalski, R., Kedzia, B (2007). Antibacterial activity of Solanum aculeastrum (Solanaceae). Pharmacol. Biol. 44: 284 – 286.
14. Lambert, J. 92001). Ethiopian traditional medicine and the bridge to better health. Indigenous Knowledge (IK) Notes. 35: 114 – 118.
15. Mbata, T. I., Duru, C. M., Onwumelu, H. A. (2009). Antimicrobial activity of 21 International Journal of Current Research and Review www.ijcrr.com Vol. 03 issue 12 December 2011 crude seed extracts of Bucholzia coriaceae on some pathogenic bacteria. Journal of Developmental Biology and tissue Engineering. 1(1): 001 – 005.
16. Nweze, N. E., Asuzu, I. U. (2006). The antihelmintic effect of Bucholzia coriaceae seed. Nigerian Vet. Journal.27 (2): 60 – 65.
17. Parekh, J., Chanda, S. (2007). In vitro antimicrobial activity of Trapa natans Linn. fruit rind extracted in different solvents. African Journal of Biotechnology. 6(6): 766 – 770.
18. Peni, I. J., Elinge, C. M., Yusuf, H., Itodo, A. U., Agaie, B. M., Mbongo, A. N., Chogo, E. (2010). Phytochemical screening and antibacterial activity of Parineri curatellifolia stem extract. Journal of Medicinal Plants Research. 4(20): 2099 – 2102.
19. Trease, G. E., Evans, W. C. (1983). Text book of Pharmacognosy (12th Ed). Balliere Tindall, London. Pp. 343 – 384.
20. Umerie, S. C., Emelugo, B. N. (2007). Phytochemical analysis and antimicrobial activity of Cyperus rotundus Linn. tubers (Nut sedge). Natural and Applied Sciences Journal. 8(2): 114 – 119.
21. Vollkova, A., Kostalova, D., Sochorova, R. (2001). Isoquinoline alkaloids from Mahonia aquifolium stem bark is active against Malassezia aquifolium spp. Folia Microbiol. 46: 107 – 111.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License