IJCRR - 4(1), January, 2012
Pages: 34-43
Print Article
Download XML Download PDF
ANTINOCICEPTIVE EFFECTS OF DIAZOXIDE IN RODENT MODEL OF CHRONIC PAIN INDUCED BY
CHRONIC CONSTRICTION NERVE INJURY
Author: Deshmukh A. B., Patel J. K., Prajapati A. R., Patel K. S., Jadav R. J.
Category: Healthcare
Abstract:Aim: Previous studies show that neuropathic pain is refractory against conventional analgesics and thus novel medicaments are desired for the treatment. Activated K+ channels are associated with reducing inappropriate or excessive neuronal activity. The aim of this study was to investigate the possible analgesic effects of potassium channel opening on neuropathic pain. Therefore, the present study was designed to investigate whether potassium channel activator can generate qualitative analgesic effects on the acute pain induced by thermal and mechanical stimulation. Methods: The effect of diazoxide at the dose of 200 mg/kg on acute thermal and mechanical nociception were assessed by sensory testing like spontaneous pain, mechanical hyperalgesia, tactile as well as cold allodynia in chronic constriction injury (CCI) induced pain in rat. Results: After CCI surgery, the rats developed neuropathic pain syndrome. Behavioral studies demonstrated that rats with the CCI experienced spontaneous pain, dynamic allodynia and mechanical hyperalgesia which were significantly different from the sham group. Treatment with diazoxide decreased significantly the withdrawal durations in all sensory tests. Conclusion: The present study indicates that activity of K+ channel may contribute significantly to the development of central sensitization-mediated pain and suggests that K+
openers may be an important molecular target for the treatment of chronic pain of neuropathic origin.
Keywords: Neuropathic pain, Diazoxide, Potassium channels, Chronic constriction injury, sensory test
Full Text:
INTRODUCTION
Neuropathic pain is one of the most significant health problems in the world. Neuropathic (neurogenic) pain is defined by IASP as pain caused by a lesion or dysfunction of the nervous system (1). It is characterized by inappropriate spontaneous or excessive neuronal activity in response to physiological stimuli. Chronic pain is one of most common reasons for hospital visits so it should be considered to be a disease rather than just a symptom. Recent advances in molecular biology techniques and the subsequent discoveries of key molecules involved in pain production, have clearly contributed to better understanding acute pain (2-5), by which the molecular multiple mechanisms underlying chronic pain can be fully clarified. Proper diagnosis and early treatment are often found to be difficult in neuropathic pain, because it is quite different from other types of pain, such as nociceptive (or physiological) or inflammatory pain and it is irreversible, even when the underlying cause has been rectified (3). Also, the occurrence of neuropathic pain is commonly as a secondary symptom in diseases (e.g. diabetes, cancer, and herpes zoster infection) or as a side effect of chemotherapeutic treatments (4, 6-8). The management of this disorder is achieved by various classes of drugs that are capable of dampening neuronal excitability. Examples may be voltagegated sodium channel blockers (carbamazepine, phenytoin, lamotrigine and topiramate), voltage-operated calcium channel modulators (ethosuximide, gabapentin, levetiracetam) and modulators of inhibitory GABAergic neurotransmission (benzodiazepines, vigabatrin and tiagabine). Of these, the approved drugs for the treatment of neuropathic pain are gabapentin, and carbamazepine and lamotrigine has demonstrated efficacy for neuropathic pain in clinical trials (9). Various drugs with sodium channel blocking actions preferentially suppress thermal nociception which may be partly explained by the local anesthetic action of sodium channel blocking agents and differential sensitivities to local anesthetics of the fibers activated by thermal and mechanical nociception (10). However, a number of issues regarding this treatment, including the effective, meaningful drug dose range, the durability of pain-relief effects leading a poor treatment to patient with currently available drugs. So there is a need for new agents with their novel mechanism for the treatment of neuropathic pain. Potassium (K+ ) channel opening is one potential mechanism that has not yet been exploited for neuropathic pain. Activated K+ channels are associated with reducing inappropriate or excessive neuronal activity (11). Therefore, the present study was designed to investigate whether potassium channel activation generate qualitative analgesic effects on the acute pain induced by thermal and mechanical stimulation.
MATERIALS AND METHODS
Animals
Wistar-kyoto male rats of 8 weeks age with the body weight range from 250-300 gms were procured from Central Animal Facility, Nootan pharmacy college, Visnagar, India. They were maintained in essential condition of controlled temperature (<30? C) and humidity (< 70%) with 12 hour day and night cycle according to the norms of CPCSEA.
Chronic Constriction Injury (CCI)
CCI surgery was carried out as described (12). Rats were anesthetized with combination of ketamine and xylazine and a 7-mm segment of the left common sciatic nerve was exposed at the mid-thigh level, Proximal to the sciatic trifurcation, about 7 mm of nerve was freed of adhering tissue and 4 ligatures (4-0 silk) were tied loosely around at about 1 mm spacing. Ligatures were tied loosely enough so that, on visual inspection, blood flow was not obstructed. The incision was closed in layers. Post seven days of healing nociceptive test was done followed by behavioural assessment.
Sham Surgery: Same surgical procedure was followed in six animals except the removal part/whole of kidney and kidneys were touched with forceps and threads.
Drugs and Chemicals:
Diazoxide was procured from Ranbaxy Research laboratories, India. DMSO was procured from Veeraj Associates Ahmedabad, India. Ketamine and Xylazine were purchased from Visnagar, India.
Grouping of animals:
After one week of CCI in animals they divided in to three groups: Group 1: sham group, Group 2: CCI with drug (Diazoxide) and Group 3: CCI with vehicle (CCI group).
Treatment with diazoxide:
Animals in group 2 were treated with Diazoxide at the dose of 200 mg/kg (dissolved in DMSO 25mg/ml) by oral route once a day for 7 days, while that of group 3 were treated with vehicle (DMSO).
Sensory testing using nociceptive assay in CCI rats:
Four nociceptive assays aimed to determine the severity of neuropathic responses namely allodynia and hyperalgesia were performed. Estimation of parameters at basal, day 1, day3, day 5 and day 7.
1. Spontaneous pain
2. Dynamic allodynia
3. Cold allodynia
4. Mechanical hyperalgesia
Spontaneous pain:
Spontaneous pain was assessed for total period of 5 min as described (13). The operated rats were placed into the observation cage 5 cm from working place. An initial acclimatization period of 10 min was given to each of rats. From each group total six rats were assessed. The test is based on recording of the cumulative duration that the rats hold its ipsilateral paw off the floor. The paw lifts associated with locomotion or body repositioning was not counted. The paw lifts in the absence of any overt external stimuli are associated with spontaneous pain, and are correlated of ongoing pain are counted.
Dynamic allodynia:
All of the operated rats were assessed for dynamic allodynic response according to the procedures described (14). The operated rats were placed into the observation cage 5 cm from working place. Lifting of the affected paw for finite period of time in response to mild stroking on the plantar region using cotton-bud is a positive dynamic allodynia. This stimulus is non-noxious to normal- behaviouring rats. The latency to paw withdrawal was then counted. If no paw withdrawal was shown within 15s, the test was terminated and animal were assigned withdrawal time. Hence 15s effectively represented no withdrawal.
Cold allodynia:
Application of cold (an acetone drop placed on the paw) in the on the plantar region (15) was used for estimating the withdrawal duration of cold allodynia. If no paw withdrawal was shown within 15s, the test was terminated and animal were assigned withdrawal time. Hence 15s effectively represented no withdrawal.
Mechanical hyperalgesia:
The operated rats were assessed for mechanical hyperalgesia sensitivity according to the procedure described (16). The initial set up was same as previous test. The measurement of hind paw withdrawal duration was done after mild pinprick stimulus to the mid plantar surface of the ipsilateral (left) hind paw. A withdrawal was defined as being abnormally prolonged if lasted at least 2s. The mean withdrawal duration was taken from a set of three responses.
Statistical analysis
All the data were expressed as mean ± s.e.m. The single treatment studies were analysed using unpaired t-test using graphPad Prism 5.0.
RESULTS
General observations
After CCI surgery, the rats developed neuropathic pain syndrome as previously described (12). Unusual gait and posture of the rats was observed as early as on the first day after surgery. The rats often raised the affected hind paws from the floor and hold them in a protected position. Frequent licks of the affected paws were seen. All the animals were in good health and the behavior was generally normal with no palsy or additional sensory dysfunction encountered. There was no significant difference among the weights of the three groups (data not shown).
Effects on Sensory testing:
Spontaneous pain:
After CCI surgery, the rats developed spontaneous pain which was found to be 191.67 ± 18.97 at the end of 7th day. There was a gradual elevation in the spontaneous pain from day 1 of surgery. Drug treatment at the dose of 200 mg/kg decreased the spontaneous pain. A gradual reduction was observed in animals of group 2 which was observed to be 58.33 ± 2.55 at the end of day 7 post treatment. There was a marked increase in the withdrawal duration in group 3 animals as the days progressed post surgery which was greatest at the end of day 7.
Dynamic allodynia:
Animals exhibited dynamic allodynia which was 72.67 ± 4.73 at the end of day 7, and was found to be reduced to 49.33 ± 0.51 in group 2. A significant difference was found in the withdrawal duration for dynamic allodynia between group 2 and group 3. Moreover sham operated animals of group 1 showed negligible duration of withdrawals, thus a significant difference was also observed between group 1 and group 3.
Cold allodynia:
Application of cold (an acetone drop placed on the paw) in the on the plantar region (15) was used for estimating the withdrawal duration of cold allodynia. If no paw withdrawal was shown within 15s, the test was terminated and animal were assigned withdrawal time. Hence 15s effectively represented no withdrawal.
Mechanical hyperalgesia:
The operated rats were assessed for mechanical hyperalgesia sensitivity according to the procedure described (16). The initial set up was same as previous test. The measurement of hind paw withdrawal duration was done after mild pinprick stimulus to the mid plantar surface of the ipsilateral (left) hind paw. A withdrawal was defined as being abnormally prolonged if lasted at least 2s. The mean withdrawal duration was taken from a set of three responses.
DISCUSSION
Painful stimuli are transferred to the CNS by the lateral spinothalamic tract. First order neurons transmitting pain impulses from the skin (Aδ and C fibres) enter the substantia gelatinosa of the dorsal horn via the dorsal roots. Second order neurons in the lateral spinothalamic tracts convey impulses associated with pain up to the nuclei of the ventroposterior thalamus where the painful impulses are integrated. From the thalamus, third order neurons convey the impulses up to the cerebral cortex, where subjective interpretation of pain is thought to occur (17). Neuropathic pains are disorders characterized by excessive neuronal activity. These disorders are currently managed by drugs that are capable of dampening neuronal excitability, including voltage-gated sodium channel blockers, voltage-operated calcium channel modulators and modulators of inhibitory GABAergic neurotransmission. However, these drugs are rarely 100% efficacious and their use is often associated with limiting side effects. Thus, there is a clear medical need for novel agents to treat these diseases. One potential mechanism that has not yet been exploited is potassium (K+ ) channel opening. A significant and growing body of genetic, molecular, physiological and pharmacological evidence now exists to indicate that KCNQ-based currents represent particularly interesting targets for the treatment of diseases such as epilepsy and neuropathic pain (11). Moreover, ATP-sensitive potassium (KATP) channels may be linked to mechanisms of pain after nerve injury, but remain underinvestigated in primary afferents so far. A study characterized these channels in dorsal root ganglion (DRG) neurons, and tested whether they contribute to hyperalgesia after spinal nerve ligation (SNL). Following SNL, this channel activity was suppressed in large neurons from hyperalgesic rats. In large neurons, selective inhibition of whole-cell ATPsensitive potassium channel current (IK(ATP)) by glibenclamide depolarized resting membrane potential (RMP). The contribution of this current to RMP was also attenuated after painful axotomy. These findings indicate that functional KATP channels are present in normal DRG neurons, wherein they regulate RMP. Alterations of these channels may be involved in the pathogenesis of neuropathic pain following peripheral nerve injury. Their biophysical and pharmacological properties are preserved even after axotomy, suggesting that KATP channels in primary afferents remain available for therapeutic targeting against established neuropathic pain (18). A study has demonstrated that KATP channel opening results in decreased excitability, attenuated neurotransmission, and possibly antihyperalgesia. Therefore, these channels in DRG neurons provide novel opportunities for therapeutic targeting using KATP channel openers (19) or CaMKII activators (20, 21) as analgesics in neuropathic pain. We tested a hypothesis that K+ channel opener like diazoxide administration may decline the pain due to decreased excitability of the hyperactive nerves and improve the pain condition to which we obtained consistent results. While our ultimate goal was to elucidate the role of K + channels in neuropathic pain, we employed a well-established chronic constriction injury model (CCI) in this study to investigate effects of our approach. CCI also known as Bennett model is a rat model of painful peripheral mononeuropathy (12). CCI rats show behavioral signs of spontaneous pain such as mild to moderate autotomy, guarding, excessive licking and limping of ipsilateral hind paw, and avoidance of placing weight on the injury side. Hyperalgesia due to noxious thermal and mechanical stimuli is detectable, as are cold allodynia and tactile (12, 22). All pain signs last for the entire duration of the study. CCI induced spontaneous pain which was characterized by signs of paw guarding, lifting, and limping, excessive grooming and biting, changes in exploratory behavior, weight bearing. In addition, evoked pain (allodynia and thermal hyperalgesia) to thermal or mechanical stimuli was observed in CCI group although it was at different levels. There was a significant increase in the all the four Sensory parameters like spontaneous pain, dynamic allodynia, cold allodynia and mechanical hyperalgesia as compared to the animals of sham group. The parameters were noted at the interval of 1 day right from basal, day 1 post surgery, day 3, 5 and 7 post surgery. There was a gradual augmentation in the withdrawal duration as was observed from the data from day 1 to day 7. No statistically significant difference was observed between recordings in sham group till day 7. Treatment with diazoxide at the dose of 200 mk/kg per orally for 7 days significantly reduced spontaneous pain in animals. Moreover a significant change in the mechanical and thermal evoked allodynia/hyperalgesia was also observed as compared to the CCI group. The development of thermal hyperalgesia and tactile allodynia is known to involve separate pathways (23). While noxious thermal stimuli is thought to be mediated through high-threshold, thin unmyelinated primary afferent C- fibers, non-noxious tactile stimulation is believed to be mediated through large diameter, low threshold Ab afferent fibers, and processed at supraspinal sites receiving input through the dorsal columns (24-26). We observed that there was retardation in the progression of the disease in drug treated group as compared to CCI group. But it was not restored to a normal value instead a partial restoration of values were obtained. More extensive research is needed in this area for more specific answers to the questions regarding the involvement of K+ channels in neuropathic pain.
CONCLUSION
Neuropathic pain is thought to become worse with time, untreated appropriately inducing a vicious circle. The development of agents that may block enhanced pain transmission is an important therapeutic approach for research. Neuropathic pains are resistant to conventional narcotic therapies and often incapacitate patients. The search for novel treatments for this pain syndrome characterized by central sensitization has stirred numerous investigations in both the basic science and clinical arenas. The present study indicates that activity of K+ channel may contributes significantly to the development of central sensitization-mediated pain and suggests that K+ openers may be an important molecular target for the treatment of chronic pain of neuropathic origin.
References:
1. Cruccu G, Anand P, Attal N, GarciaLarrea L, Haanp M, Jorum E et al. EFNS guidelines on neuropathic pain assessment. European Journal of Neurology 2004; 11 (3): 153–62
2. Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron 2007; 55: 353-64.
3. Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci 2002; 5 Suppl: 1062-67.
4. Ueda H. Molecular mechanisms of neuropathic pain-phenotypic switch and initiation mechanisms. Pharmacol Ther 2006; 109: 57-77.
5. Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 2007; 55: 365-76.
6. Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006; 52: 77-92.
7. Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 1999; 353: 1959-64.
8. Matsumoto M, Inoue M, Hald A, Xie W, Ueda H. Inhibition of paclitaxelinduced A-fiber hypersensitization by gabapentin. J Pharmacol Exp Ther 2006; 318: 735-40.
9. Eisenberg E, Lurie Y, Braker C, Daoud D, Ishay A. Lamotrigine reduces painful diabetic neuropathy: a randomized, controlled study. Neurology 2001; 57: 505-9
10. Sakaue A, Honda M, Tanabe M, Ono H. Antinociceptive Effects of Sodium Channel-Blocking Agents on Acute Pain in Mice. J Pharmacol Sci 2004; 95: 181–88.
11. Wickenden AD, Roeloffs R, McNaughton-Smith G, Rigdon GC. KCNQ potassium channels: drug targets for the treatment of epilepsy and pain. Expert Opin. Ther. Patents 2004; 14(4): 1-13.
12. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988; 33: 87- 107.
13. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994; 59: 369–76.
14. Field M J, Scott M, Hughes J, Singh L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain 1999; 80: 391–98.
15. Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000; 87: 149–58.
16. Field MJ, Bramwell S, Hughes J and Singh L. Detection of static and dynamic components of mechanical allodynia in rat models of neuropathic pain: are they signalled by distinct primary sensory neurones? Pain 1999; 83: 303–11.
17. Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat. Rev. Neurosci. 2001; 2(2): 83-91.
18. Kawano T, Zoga V, Mccallum JB, Wu HE, Gemes G, Liang MY et al. ATPsensitive potassium currents in rat primary afferent neurons: biophysical, pharmacological properties, and alterations by painful nerve injury. Neuroscience 2009; 162(2): 431-43.
19. Sachs D, Cunha FQ, Ferreira SH. Peripheral analgesic blockade of hypernociception: Activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc Natl Acad Sci USA 2004; 101: 3680–85.
20. Sawynok J, Esser MJ, Reid AR. Peripheral antinociceptive actions of desipramine and fluoxetine in an inflammatory and neuropathic pain test in the rat. Pain 1999; 82: 149–58.
21. Tiraboschi E, Giambelli R, D'Urso G, Galietta A, Barbon A, de Bartolomeis A. Antidepressants activate CaMKII in neuron cell body by Thr286 phosphorylation. Neuroreport 2004; 15: 2393–96.
22. Attal N, Jazat F, Kayser V, Guilbaud G. Further evidence for „pain-related? behaviours in a model of unilateral peripheral mononeuropathy, Pain 1990; 41: 235–51.
23. Ossipov MH, Lai J, Malan Jr TP, Porreca F. Spinal and supraspinal mechanisms of neuropathic pain. Ann NY Acad Sci 2000; 909: 12–24.
24. Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain 1996; 68: 133–40.
25. Ossipov MH, Bian D, Malan Jr TP, Lai J, Porreca F. Lack of involvement of capsaicin sensitive primary afferents in nerve-ligation injury induced tactile allodynia in rats. Pain 1999; 79: 127–33.
26. Willis WD, Al-Chaer ED, Quast MJ, Westlund KN. A visceral pain pathway in the dorsal column of the spinal cord. Proc Natl Acad Sci USA
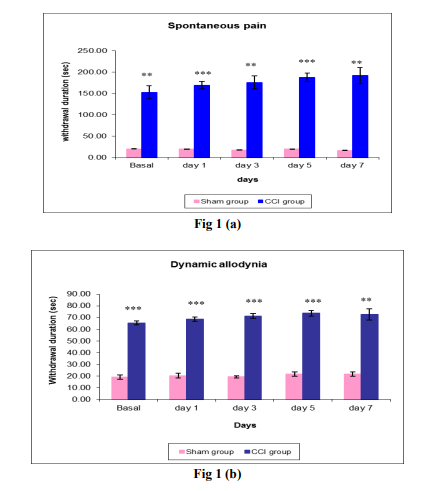
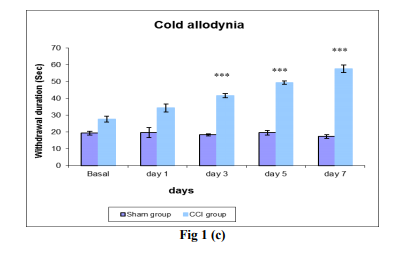


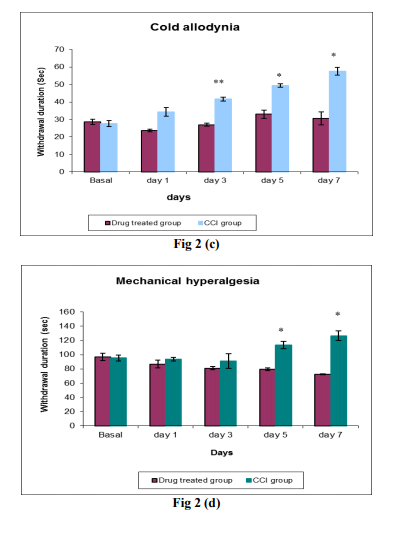
Fig 1: Comparison of withdrawal duration (sec) in sham group and CCI group at the basal, day 1, 3, 5, and 7 days post surgery by sensory tests like (a) Spontaneous pain, (b) Dynamic allodynia, (c) Cold allodynia and (d) Mechanical hyperalgesia. Each data point represents the mean ± S.E.M. The significance of differences between the sham group and CCI group values was determined by unpaired t-test. *P<0.05 and **P<0.01, ***P<0.001 vs sham group in respective time.
Fig 2: Effect of Diazoxide (200mg/kg, p.o. once a day ) treatment for 7 days in CCI induced chronic pain at the basal, day 1, 3, 5, and 7 days by sensory tests like (a) Spontaneous pain, (b) Dynamic allodynia, (c) Cold allodynia and (d) Mechanical hyperalgesia. Each data point represents the mean ± S.E.M. The significance of differences between the drug treated group and CCI group values was determined by unpaired t-test. *P<0.05 and **P<0.01, ***P<0.001 vs sham group in respective time
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License