IJCRR - 4(1), January, 2012
Pages: 22-28
Print Article
Download XML Download PDF
IN VITRO EVALUATION OF AQUEOUS AND ETHANOLIC EXTRACTS OF VERNONIA COLORATA AS
AN ANTIBACTERIAL AGENT
Author: Oseni, Lateef Adebayo, Berkoh, Eric Asamoah, Mills-Robertson, Felix Charles
Category: General Sciences
Abstract:Recent reports have shown the increased emergence of bacteria resistance to many existing antimicrobial drugs. This has prompted the need to find alternative remedies, and plant products have proven to be vital in this search. Vernonia colorata has been reported to be active against syphilis, pneumonia, measles, dysentery and several skin infections in traditional medical practices. In the present study, aqueous and ethanolic extracts from the leaves of Vernonia colorata were evaluated in vitro for growth inhibitory activity on Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Staphylococcus aureus using Agar diffusion method. Phytochemical analysis revealed the presence of reducing sugars, saponins, polyphenols, tannins, phlobatannins, alkaloids, sterols and triterpenes in both extracts. These classes of phytochemicals have widely been reported for their antibacterial properties. Of the several bacteria tested, only S. aureus and P. aeruginosa showed significant susceptibility to both ethanolic and aqueous extracts with concentrations range between 2.00 to 5.00mg/ml. The aqueous extract also showed the highest activity on S. aureus at concentration of 5mg/ml. The Minimum Inhibitory Concentration of the aqueous extract ranged between 4.00 and 6.00mg/ml while that of the ethanolic extract ranged between 5.00 and 6.00mg/ml. Following the results from the current study, it can be concluded that V. colorata has significant antibacterial activity and will be very useful in the discovery of novel antibiotics against S. aureus and P. aeruginosa
Keywords: Vernonia colorata, Antibacterial activity, Minimum Inhibition Concentration, Phytochemical screening, Agar diffusion method
Full Text:
INTRODUCTION
Both ancient and modern men of all cultures have widely used medicinal plants for treating different ailments. Scientific discoveries have shown that plants produce a wide range of complex compounds (secondary metabolites) as part of their normal metabolic process. Several secondary metabolites have been reported to have significant therapeutic properties. Therefore, plants are model source of medicines as they contain many chemical agents with therapeutic properties. Despite increasing advancement in the field of medicine and molecular diagnosis, reports indicate that close to 80% of the world population still dependant on plant derived pharmaceuticals. There are also reports that suggest that nearly 28% of drugs available in the market are plant based products and its derivatives. (Newman et al., 2003). In recent times, scientists have extensively reported on bio-assays of several plants of nutritional and medicinal values. Furthermore,a large proportion of compounds used as lead molecules in drug discovery are plant based compounds. This suggests that plant based compounds play a vital role in diversity oriented synthesis of natural product-like pharmaceuticals. (Marcaurelle and Johannes, 2008). Bacterial infection treatment options include chemotherapy, radiation therapy and surgery. In chemotherapy, antibacterial drugs are usually employed in the treatment of various forms of bacterial infections. However, there are reports on increased emergence of multiresistant bacterial strains of clinically important pathogens. This development has fetched the interest of scientist to develop newer broad spectrum antimicrobial agents. Due to the high cost and the less availability of new generation antibiotics, it is imperative to look for the substances from alternative medicines with claimed antimicrobial activity. A significant number of medicinal plants with significant antimicrobial activity have been reported in different traditional literatures. Vernonia is a genus of about 1000 species of forbs and shrubs in the family Asteraceae. The uses of several species of Vernonia, including Vernonia amygdalina, Vernonia auriculifera, Vernonia colorata, Vernonia galamensis, and Vernonia hymenolepis in traditional health care have been reported. There are many convergence in the usage of Vernonia spp. in its traditional use throughout West and Central Africa and North America as anti-inflammatory, analgesic, antibacteria, anticancer, antidiabetic, antifungal, antimalaria and antioxidant.(Abosi and Raseroka, 2003; Phillipson et al., 1993). Previous studies on Vernonia spp have largely been confined to Vernonia amygdalina. This species has been reported to contain glycosides, tannins, steroidal saponins, sesquiterpenes lactones flavonoids and vitamin C. (Ifeoma and Chukwunonso., 2011). V. Amygdalina is also known for its activity against syphilis, malaria, measles, dysentery and yellow fever. (Oluwalana and Adekunle.,1998). V. colorata has also been reported to be used in traditional herbal medicine across many African countries for the treatment of bacteria, fungal, parasitic and inflammatory disorders. Despite the traditional uses of V. colorata in primary health care, reports on the phytochemical profile and bio-assay of V. colorata are limited. In this regard, we aim to explore scientifically, the antibacterial potential of V. colorata to substantiate the reported claims. The current research seeks to screen for phytochemicals in the leaves of Vernonia colorata and also evaluate its potential as an antibacterial agent by analysing its growth inhibitory activity on E. coli, K. Pneumoniae, P. aeruginosa and S. aureus.
MATERIALS AND METHODS
Materials
Plant material
Fresh leaves of vernonia colorata were collected from a piece of land at MampongAkuapim in the Eastern Region of Ghana. The leaves were later taken to the herbarium Department of the Centre for Scientific Research into Plant Medicine, MampongAkuapim, for botanical identification.
Reagents
Ethanol, Fehling?s solutions, Chloroform, Acetone, Sodium Picrate paper, Ferric chloride, Ammonia, Chloramphenicol test paper, Dimethyl sulphoxide (DMSO), Hydrochloric acid (HCl), and Sulphuric acid(H2SO4) were of analytical grade and from BDH, UK. Other reagents used were of analytical grades and water used was glass distilled.
Bacteria strains
Four different microbes of standard strains were purchased at the Komfo Anokye Teaching Hospital (KATH) Kumasi, with standard codes. The microbes were; E.coli (ATCC25922), K. Pneumoniae (ATCC 33495), P. aeruginosa (ATCC 27853) and S. aureus (ATCC 25923).
Methods
Extraction from plant material
Adequate quantity of the leaves were collected, sun dried and then milled. The fine powder was then divided into two portions. Preparation of ethanolic extract About 500g of the powder were soaked in 5L of 70% ethanol for 24 hours. The suspension was then filtered. The filtrate was concentrated using rotary evaporator and then freeze dried. Preparation of aqueous extract Another 500g sample of the powdered leaves was soaked in 5L of distilled water and then boiled for 15minutes after which the temperature was lowered to 60oC for another 15 minutes. This was then filtered and the filtrate evaporated using rotary evaporator at 60oC and then freeze dried.
Phytochemical analysis
About 5g of each freeze dried sample of crude extract was dissolved to 100ml and portions analyzed for phytochemical constituents using standard methods.(Stahl , 1969; Harborne, 1973)
Microbiology
Media
Muller Hilton Agar and Peptone Agar were used.
Sterilization
All other materials used in the microbiological work were sterilized before usage.
Preparation of Muller Hilton agar
About 15.2g of Muller Hilton agar was weighed and added to 400ml of distilled water, and then heated to dissolve. The media was sterilized at 121oC in an autoclave for 20 minutes and then cooled to about 60oC. About 20ml of the media was poured gently into each plate and left to cool and then stored in an oven at 370C for 16 hours.
Peptone water
Peptone agar was used as the broth for the culture of the microbes About 0.5g of peptone agar was weighed and added to 50ml of distilled water and then heated to dissolve. This was sterilised at 121°C for 20 minutes and cool to about 60°C. 5ml portions of the broth were pipetted into test tubes and then stored in an oven at 370C for 16 hours.
Antimicrobial susceptibility test
The antibacterial test was performed using the agar diffusion method of Collins et al. (1995). The test microorganisms were inoculated on nutrient agar plates and spread uniformly using a sterile glass spreader. Wells of 5 mm in diameter were made on the nutrient agar using a sterile cork borer. The cut agar disks were carefully removed by the use of forceps sterilized by flaming. Different concentrations of the freeze extracts were prepared by dissolving various masses in 20% dimethyl sulphoxide (DMSO). To each well was introduced different concentrations (1.0, 2.0, 3.0, 4.0, 5.0 mg/ml) of plant extracts. Control experiments were set up using Chloramphenicol and DMSO as positive and negative controls respectively.
The plates were allowed to stand for one hour at room temperature for diffusion of the substances to proceed before the growth of microorganisms commenced. The plates were made in triplicate and were incubated at 37°C for 24 h. Diameters of zones of inhibition in the triplicate plates were measured by calculating the difference between cork borer (5mm) and the diameters of inhibition (Hewett and Vincent, 1989; Singh et al., 2002; Adebayo and Adegoke; 2009). The zones of inhibition were then recorded.
Determination of minimum inhibitory concentration (MIC)
Various concentrations of both aqueous and ethanolic extracts ranging between 4.0 and 6.0 mg/ml were introduced into different test tubes; each tube was inoculated with an overnight culture of S. aureus, P. aeruginosa, E. coli and K. pneumonia diluted to give a final concentration of 106 cells per ml. The tubes were incubated at 37°C for 24 h. The least concentration of extract that did not permit any visible growth of the inoculated test organism in broth culture was regarded as the minimum inhibitory concentration (MIC) in each case (Collins et al., 1995).
RESULTS AND DISCUSSION
Phytochemistry of plant extracts
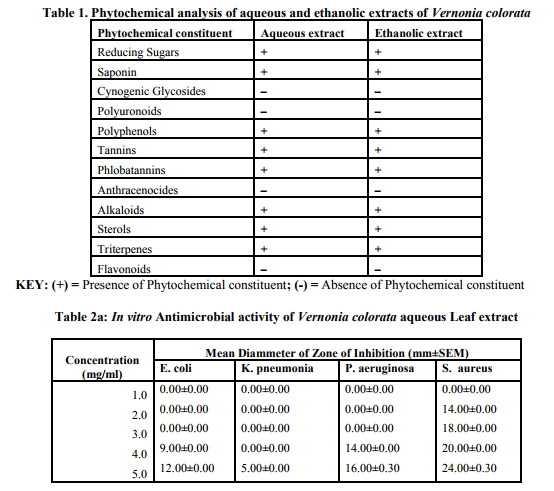
Preliminary phytochemical screening of crude aqueous and ethanolic extracts from leaves of V. colorata revealed the presence of triterpenes, sterols, alkaloids, reducing sugars, polyphenols, tannins, phlobatannins and saponins. Cynogenic glycosides, polyuronoids, anthracenocides and flavonoids were absent.
(Table1).
Antimicrobial activity
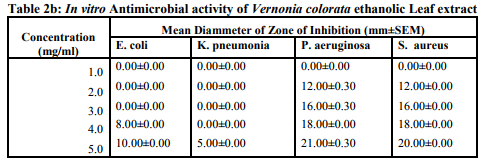
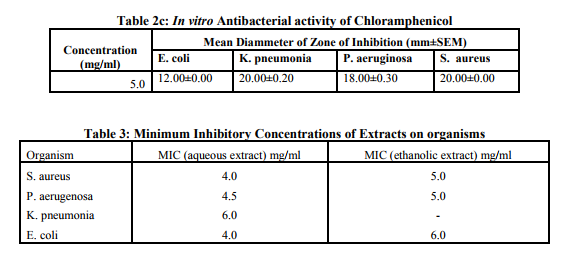
Both crude ethanolic and aqueous forms of the extracts of V. colorata exhibited varying degree of antimicrobial activities against the test organisms. (Table 2a,b) E. Coli and K. pneumoniae showed significant resistance against both aqueous and ethanolic extracts of V. colorata at concentrations below 5.00mg/ml. However, S. aureus and P. aerugenosa showed susceptibility to both extracts at concentrations between 4.00 and 5.00mg/ml. (Table 2a,b). It was observed that antibacterial effectiveness increased with increasing concentration of extracts. This supports previous work by Kurosaki and Nishi (1983) who reported that higher concentrations of antimicrobial substances showed appreciable growth inhibition to microorganisms. Although, phytochemical screening revealed similar results for both aqueous and ethanolic extracts, the compounds found in the classes of phytochemicals present in each extract may differ and this could account for their varying inhibitory activities. (Table 2a,b). Another possible reason for the varying degree of inhibition by the extracts could be the presence of other classes of phytochemicals that were not tested for in the ethanolic extract. Further to this, the boiling of leaves during the preparation of the aqueous extract may render some compounds inactive in the aqueous extract and this could also contribute to the observed variation in inhibitory activities. Although the positive control (Chloramphenicol) showed significant growth inhibitory activity on all the bacteria tested, the aqueous extract was found to be more effective on S. Aureus at concentration of 5mg/ml while the ethanolic extract was found to more effective on P. Pneumonia at the same concentration than the standard.(Table 2c). The MIC of the aqueous extract in this study against the test organisms ranged between 4.0 and 6.0 mg/ml while those of the ethanolic extract ranged between 5.0 and 6.0 mg/ml. (Table 3). Antimicrobial agents with low activity against an organism have a high MIC while a highly active antimicrobial agent gives a low MIC. The present result shows that the aqueous extract is slightly more effective than the ethanolic extract. Tannins, alkaloids saponins and phlobatannins have been reported for their antibacterial and antiviral activity (Enzo, 2007). Furthermore, alkaloids and saponins are classes of compounds that are known to be effective for the treatment of syphilis and other venereal diseases. (Sofowara, 1993). Steroids in modern clinical studies are known for their antiinflammatory and analgesic properties. (Pithayanukul et al., 2007). The antibacterial activities demonstrated by extracts from V. colorata may be attributed to the presence of these phytochemicals and this supports the use of the plant for the treatment of syphilis, pneumonia and skin infections as reported in traditional folk medicine.
CONCLUSION
Although, a large number of medicinal plants are constantly screened for their antimicrobial effects, many plant species with potent antimicrobial properties are yet to be discovered. The present study reveals the antibacterial potential of leaves of V. colorata. The antibacterial activities of the extracts against S. aureus and P. aeruginosa were comparable to those of the standard (Chloramphenicol). These results seem to justify the continued use of the plant in the treatment of microbial infections such as pneumonia, syphilis and skin diseases. In addition, the inhibition of growth of the test organisms (S. aureus and P. aeruginosa) that are known to display multidrug resistance to most antibiotics and nonantibiotic antimicrobial agents justify the continued use of these plants in folk and traditional medical practice.
References:
1. Abosi, A.O. and Raseroka, B. H. (2003). In vivo anti-malarial activity of Vernonia amygdalina. Br. J. Biomedical Sci. 60: 89 -91.
2. Adegoke, Anthony A. and Adebayo-Tayo, Bukola C. (2009). African Journal of Biotechnology Vol. 8 (1), pp. 077-080
3. Collins GH, Lynes PM, Grange JM (1995). Microbiological Methods (7th edn) Butterwort – Heinemann Ltd, Britain pp. 175–190.
4. Enzo, A.P., (2007). Traditional plants and herbal remedies used in the treatment of diarrheal diseases. Mode of action, quality, efficacy and considerations. In: Ahmad I, Aqul F, Qwaiss M, Modern Phytomedicine Turning Medicinal Plants into Drugs. WILEY-VCH Verlag GMBH and Co. KGQA. Weinheim, pp: 248-260.
5. Harborne J.B. Phytochemical methods (1984). 2nd ed. Chapman and Hall, New York, 3:100-117.
6. Harborne J.B. Phytochemical methods (1984). 2nd ed. Chapman and Hall, New York, 1:4-7.
7. Harborne JB. Phytochemical Methods. Chapman and Hall, London (1973) 172- 278.
8. Hewitt W, Vincent S (1989). In:Theory and application of microbiological assay. Academic Press, San Diego, p. 39.
9. Ifeoma I. Ijeh and Chukwunonso E. C. C. Ejike (2011). Current perspectives on the medicinal potentials of Vernonia amygdalina Del. Journal of Medicinal Plants Research Vol. 5(7), pp. 1051-1061
10. Kurosoki F, Nishi A (1983). Isolation and antimicrobial activity of the phytoalexin– 6-methoxymellein from cultured carrot cells. Phytochemistry 22(3): 669-672.
11. Marcaurelle LA and Johannes CW (2008) Application of natural product-inspired diversity-oriented synthesis to drug discovery. Prog Drug Res. 66(187):89- 216.
12. Newman DJ, Cragg GM and Snader KM (2003) Natural products as sources of new drugs over the period 1981-2002. J Nat Prod 6:1022-37.
13. Oluwalana SA, Adekunle MF (1998). Forest plant roots in household nutrition and health care J. Trop. For. Resources 14(1): 120 - 136.
14. Phillipson JD, Wright CW, Kirby GC, Warhurst DC (1993).Phytochemistry of some plants used in traditional medicine for the treatment of protozoal diseases. Abstracts, Int Symposium of the Phytochem Soc of Europe; University of Lausanne, Switzerland, p.L3.
15. Pithayanukul P., Tubprasert J., Wuthiudomlert M (2007). I n vitro antimicrobial activity of Zingiber cassumunar (Plai) oil and a 5% Plai oil gel. Phytotherapy Research, 21:164-169.
16. Singh B, Sahu PM, Sharma MK (2002). Anti-inflammatory and Antimicrobial activities of triterpenoids from Strobilanthes callosus Nees. Phytomed. 9:355-359.
17. Stahl E. (1969) Thin Layer Chromatography. 2nd ed. MRF Ashworth Springer-Verlag, Heidelberg 421-470.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License