IJCRR - 4(3), February, 2012
Pages: 130-140
Print Article
Download XML Download PDF
MUTATIONAL ANALYSIS OF INTERFERON-GAMMA GENE IN INDIAN WOMEN WITH FEMALE GENITAL
TUBERCULOSIS
Author: Venkanna Bhanothu, Jane Theophilus, Roya Rozati
Category: Healthcare
Abstract:FGTB is usually a silent disease evidencing itself only when really looked for. It usually affects females of reproductive age group. Disruption of the IFN-gamma gene in mice infected with M. tuberculosis has resulted in exacerbation of disease, progressive and widespread tissue destruction and necrosis with numerous bacteria. We therefore proposed, to study the possible association of IFN-γ gene polymorphism in Indian women with female genital tuberculosis. It is a prospective case-control study. Screening of genomic DNA samples were carried out from clinically definite 106 FGTB
patients and 100 unaffected patients aged between 18 to 40 years. +874 (T?A) IFN-γ genotyping was carried out by using sequence specific primer polymerase chain reaction (SSP-PCR) method. Statistical tests were performed using pantaray software systems. According to our investigation, FGTB patients showed more or less similar TT (30.18% vs. 30.0%), higher AA (19.81% vs. 9.0%) genotypes compared to controls and the frequency of AT genotypes decreased significantly. Distribution of IFN- γ genotypes between patients and controls were have statistical disparity. This study suggests that IFN-γ +874 T to A polymorphism have an etiological association with susceptibility of female genital tuberculosis.
Keywords: Mycobacterium tuberculosis; Interferon-? gene polymorphism; Female Genital Tuberculosis (FGTB)
Full Text:
INTRODUCTION
Female genital tuberculosis (FGTB) is usually a symptom-less disease diagnosed during investigations for infertility (Namavar Jahromi et al., 2001). It represents 15-20% of extra pulmonary tuberculosis (Rajamaheshwari, 2009). In 80-90% cases, FGTB affects women between 18-38 years of age with menstrual irregularities accounting for nearly 27% of manifestations of FGTB (Chakrabarti et al., 1998). Primary infection may occur when the male partner has active genitor-urinary TB and transmission takes place by sexual intercourse (Richards and Angus, 1998). It is usually a result of reactivation of a silent bacillemia, primarily from lungs and also thought to be from cervical TB infections (Richards and Angus, 1998; Sutherland et al., 1982). The seeding of bacilli usually occurs immediately after puberty as blood supply to the pelvic organs increases and as a result, more bacilli can reach genital organs and infect them (Crofton et al., 1992). Infection of vulva, vagina and cervix may result from direct inoculation and ascending spread to other genital organs may occur (Haas et al., 2002). The incidence of infertility in genital TB worldwide varies from 44-74%; in India it is reported to be 58% (Dam et al., 2006) and majority are in the same age group (Crofton et al., 1992). In western countries the incidence of FGTB is estimated to be <1%, whereas in some African and Asian countries it reaches 15–19% (Punnonen et al., 1983; Giannacopoulos et al., 1998). Recently, Jindal et al., 2010 have been suggested the use of Endo TB-PCR for high specificity and early diagnosis of female genital tuberculosis as performed by laparoscopy and so laparoscopy can be avoided in TB-PCRpositive patients for diagnosis keeping the PCR-negative cases in mind (Majumdar and Satwik, 2011). Due to its rarity and mild clinical picture, the index of suspicion for the diagnosis of FGTB among gynecologists is usually low. Some times, immune and genetic susceptibility of host may not help in early detection by Endo TB-PCR. Therefore, remains to be an increasing public health concern worldwide. Mycobacterium tuberculosis (M. tuberculosis) is a facultative intracellular pathogen capable of producing both a progressive disease and an asymptomatic latent infection (Parrish et al., 1998). The role of IFN gamma (IFN- γ) as the main macrophageactivator Th1 cytokine has been clearly established in animal models infected with M. tuberculosis by Flynn et al. and Dalton et al., in 1993. In human, single nucleotide polymorphisms (SNPs) located in the first intron of the IFN-γ gene (at position +874) has shown variable associations with disease susceptibility and severity (Pacheco et al., 2008). On other hand, several studies have demonstrated that ethnicity and cytokine polymorphism plays a significant role in the susceptibility to a wide range of diseases (Hoffmann et al., 2002; Newport et al., 1996) including FGTB. IFN-γ modulates a number of functions in addition to MHC expression, including the activation of macrophages, NK cells and the inhibition of the Th2 phenotype in T cells (Maher et al., 2007). Exactly which function is undermined in +874A individuals have not been determined yet. Pravica et al., 2000 noted a novel single nucleotide polymorphism (SNP), T to A, located at the +874 position from translation start site in the first intron of IFN-γ gene, which coincides with a putative NF-κB binding site that could play a fundamental role in the induction of constitutively high IFN-γ production. The differences in the magnitudes of the responses that were seen may reflect the environment in which the cohorts live, or they may reflect the nature of the patients‘ infections (Cahn et al., 2003). Disruption of the IFN-gamma gene in mice infected with M. tuberculosis has resulted in exacerbation of disease, progressive and widespread tissue destruction and necrosis with numerous bacteria (Dalton et al., 1993). Therefore, alteration in IFN-γ production may influence the susceptibility to FGTB and this alteration could be due to gene polymorphism. The homozygous T/T, A/A and heterozygous A/T alleles are associated with any increase or decrease in production of IFN-γ, this cytokine can affect the outcome of the disease severity. We therefore, hypothesized that the IFN-γ +874T/A gene polymorphism might be associated with female genital tuberculosis. This gene was chosen due to its essential central regulator role in response to infection that may be involved in FGTB pathogenesis and their potential regulation on gene expression.
MATERIALS AND METHODS
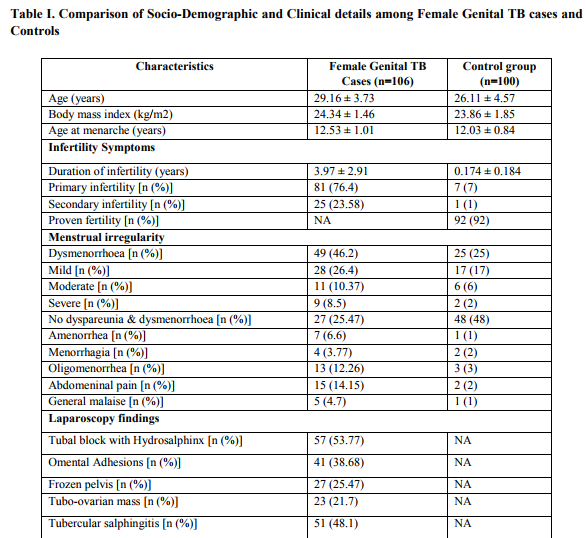
This is a prospective case-control study, which recruited women visiting the gynecology clinics at two collaborating centers, which register cases from all over the region of Andhra Pradesh, India, complaining for infertility and suspected of having genital tuberculosis (TB) on clinical grounds. During the period of our study (2006–2011), the samples from the consecutive women in these two centers were analyzed. The ethical committee of Hospital and Research Centre approved the research protocol. Informed written consents were obtained from all the participants. Proforma to obtain information on the general, obstetric and gynaecological details including family history, marital status, age at menarche, length of menstrual cycle, associated symptoms, duration and amount of blood loss, duration of infertility, and socio demographic details like social status, occupation, lifestyle, age, body mass index (BMI), limited information on diet was used and a thorough clinical examination done. General characteristics for all patients were recorded in the medical chart. Apart from routine hematological investigations, specialized investigations consisting of transvaginal sonography of uterus and adnexa, hormone profiles, immunological assays, and endoscopy were performed as and when needed. Surgically removed tissue was taken from both groups for laboratory examination, including AFB using light microscopy following concentration, staining by Ziehl–Neelsen stain as well as culture and M. tuberculosis specific PCR (Abebe et al., 2004), the diagnostic criteria by which tuberculosis was confirmed. All patients met the inclusion criteria: 18-40 years of age having irregular periods, with past history of having Genital TB and Tubal blockage, experiencing infertility (in >60% of cases), pelvic pain and scanty menstruation and amenorrhoea, and histopathological evidence in biopsy of premenstrual endometrial tissue or demonstration of tubercle bacilli in culture of menstrual blood or endometrial curetting. Exclusion criteria were as all the following: Women above 40 years of age, symptoms suggestive of pulmonary TB/Extra pulmonary TB except infertility, with normal abdomenal and vaginal examinations, other chronic disease, pregnancy or nursing, severe psychiatric dysfunction, multiple sclerosis or other autoimmune disorders, pulmonary infections, HIV co-infection, women with diabetes, malnutrition and other medical disorders like hypertension were excluded. Details of laparoscopy findings like unilateral or bilateral tubal block with hydrosalphinx, omental adhesions, frozen pelvics, tubo-ovarian masses, tubercular salphingitis and tubercles were noted in Table 1. Symptoms included pelvic pain, irregular menstrual bleeding, scanty menstruation, dysmenorrhoea oligomenorrhea, amenorrhea and infertility. A pelvic mass in variable combination aroused a suspicion. Constitutional symptoms such as sweating, increase in temperature and weight loss were not major complaints while local organ dysfunction manifested in amenorrhea, omental adhesions and bilateral tubal blockage seen on hysterosalpingographic study. The median age of the subjects was 29 (range 18- 40) years. All subjects were HIV negative and normal for pulmonary TB on the basis of complete history, physical examinations; chest X-ray, lung plain X-ray and by appropriate tests such as tuberculin test, sputum smears and sputum cultures (Raut et al., 2001; Saracoglu et al 1992). The study population is from the state of Andhra Pradesh, which is known for ethnic variations.
Study group: Tube ovarian biopsy was taken from 106 women during laparoscopy; from 45 women endometrium was obtained by curettage and 61 women with biopsy for smear microscopy, histopathology, culture and PCR for mycobacterium. All these women were infertile: primary infertility in 81 (76.4%) women and secondary infertility in 25 (23.58%) women with mean age of 29.16 ± 3.73 years, mean age at menarche of 12.53 ±1.01 years, mean duration of infertility of 3.97 ± 2.91 years. Other gynaecological pathology like dysmenorrhoea in 49 (46.2%) women, tubal block with hydrosalphinx in 57 (53.77%) women, omental adhesions in 41 (38.68%) and tubercular salphingitis in 51 (48.1%) were reported. Blood samples were collected in heparinised tubes. The specimens were received and preserved in 10% formalin, processed in routine manner and embedded in paraffin wax. Three-micron thick sections were cut and stained by haematoxylin and eosin (Namavar Jahromi et al., 2001). The diagnosis was undertaken on morphological grounds (Raut et al., 2001). Erythrocyte sedimentation rate (ESR) was performed on all the patients, which showed readings of between 57 and 123. Tissue specimens were examined by a pathologist for granulomatous reactions, fibrosis suggestive of mycobacterium disease.
Control group: Out of one hundred women who attend the same clinic for other gyaecological disorders and tubal sterilization, 92 (92%) women were proven fertile. Eight (8%) women were infertile: primary infertility in 7 (7%) women, secondary infertility in 1 (1%) women with other gynaecological pathology (3 polycystic ovaries, 1 idiopathic infertility, 4 pelvic inflammatory disease) and were laparoscopically confirmed to be without female genital tuberculosis. All the women in this group were asymptomatic with mean age of 26.11 ± 4.57 years, mean age at menarche of 12.03 ± 0.84 years and mean duration of infertility of 0.174 ± 0.184 years. The following symptoms were also present in the control group: abdominal pelvic pain was observed in 2 (2%) women, dysmenorrhoea in 25 (25 %), oligomenorrhoea in 3 (3%), there were 2 (2%) mild menorrhagia, and 1 (1%) general malaise cases. There were no severe cases as shown in the Table 1.
Culture
Homogenized samples were cultured on Lowenstein Jensen egg medium for acid-fast bacilli and incubated for 3 to 8 weeks. ZiehlNeilsen staining was used to identify the bacilli (Abebe et al., 2004).
DNA Preparation Five ml of whole blood from patients and controls were used for DNA extraction by a modified proteinase-K/salting-out method (Miller et al., 1988). Polymorphism at position +874 of IFN-γ gene was identified using sequence specific polymerase chain reaction (PCR-SSP) as described by Pravica with some modifications (Pravica et al., 2000). Briefly, red blood cells were lysed using cold Lysis buffer-I (0.3 M sucrose, 10mM Tris-HCl (pH: 7.4), 5 mM MgCl2, 1% Triton x-100). The pellet was washed with phosphate buffer saline (PBS) once. To the pellet 3 ml Lysis buffer-II (10mM Tris-HCl, 400mM NaCl, 2mM Na2- EDTA), 200μl of 10% SDS and 40μl proteinase-K were added and incubated in 37°C overnight. To remove proteins, 1 ml of 6M NaCl was added and centrifuged for 5 min at 1500g. For extraction of DNA, 2 volumes of absolute ethanol were added to the supernatant. The extracted DNA was washed twice in 70% ethanol, dried at 37°C, and recovered in sterile water. Extracted DNA was stored at -20°C until utilization.
PCR-SSP Amplification IFN-γ polymorphism at position +874 in the first intron (T versus A) was determined by sequence-specific primer-PCR (PCR-SSP) according to manufacturer‘s recommendations (QPS Bioserve India (P) Ltd, Hyderabad, India). Briefly, the PCR was performed in a final volume of 50μl with 100-200ng of isolated genomic DNA as template in reaction mixture containing 200μM (each) dNTPs and 0.5 U Taq DNA polymerase, 1X reaction buffer (Bangalore Genie, Bangalore, India), 3.5mM MgCl2 (GENETIX, New Delhi, India), 0.5μM each specific primers (antisense: TCA ACA AAG CTG ATA CTC CA; sense +874 T: TTC TTA CAA CAC AAA ATC AAA TCT; or sense +874A: TTC TTA CAA CAC AAA ATC AAA TCA), and 0.2 μM of each internal control primers (QPS Bioserve India (P) Ltd, Hyderabad, India). Internal control primers amplify a human β-globin sequence (forward primer: ACA CAA CTG TGT TCA CTA GC; reverse primer: CAA CTT CAT CCA CGT TCA CC). PCR amplification was performed using a touch down method that included initial denaturation at 95oC for 5 minutes followed by two loops; loop 1 which consisted of 10 cycles with the following program: 95oC for 30 seconds, 62oC for 50 seconds, and 72oC for 40 seconds and loop 2 included 20 cycles with the following program: 95oC for 30 seconds, 56oC for 50 seconds and 72oC for 40 seconds and a final extension step at 72oC for 5 minute. The amplified products were run on 1% agarose gel that was in a buffer containing 0.5 μg/ml ethidium bromide (Figure-1). Later it was visualized under UV light and photographs were documented.
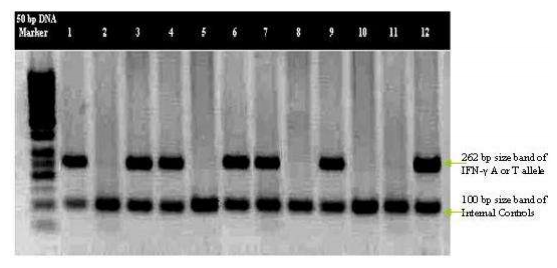
Figure-I: PCR-SSP Amplified product of IFN-γ (+874 T→A) gene SNP from FGTB patients were electrophoreses on 1% agarose gel; 262 bp size bands correspond to IFN-γ A or T allele and the 100 bp size bands correspond to internal controls. 50bp DNA marker is loaded in first well; lanes 1 and 2 show homozygosity for T allele; lanes 3 and 4 show heterozygosity for A and T alleles; lanes 5 and 6 show homozygosity for A allele; lanes 7, 8, 9 and 10 show homozygosity for T allele; lanes 11 and 12 show homozygosity for A allele.
STATISTICAL ANALYSIS
Statistical analysis was performed using pantaray software systems (Uitenbroek and Daan, 1997). Comparison of age, menarche age, body mass index, duration of infertility in the study groups and control group was performed using the independent two-sample Student‘s t test and data are presented as mean ±SD. The odds ratio (OR) and p-values were used to measure the strength of the association between genotypes and female genital tuberculosis. Hardy– Weinberg equilibrium (HWE) analysis was performed to compare genotypes frequencies between patients and controls by using χ2 analysis (df=1). All odds ratios (OR) were calculated as estimates of the confidence intervals (CI) were calculated at the 95% level (95% CI). p -value <0.05 is considered significant.
RESULTS
A total of 206 women were enrolled in the study. Symptoms are found mild and local, such as abdominal pain or menstrual irregularities, tubal blockage, tubercular salphingitis and infertility are the most common consequences (Namavar Jahromi et al., 2001), clinical signs of the FGTB patients (case group) versus control groups were given in the Table 1. Once fibrosis is established, fertility is generally difficult to restore even with appropriate treatment (Lamba et al., 2002). Therefore, this prospective large casecontrol cohort study was commenced for the SNPs of IFN-γ +874T/A in Indian women with FGTB (n=106) for the first time along with 100 controls. The 106 (51.45%) of FGTB patients were confirmed to have the evidence of M. tuberculosis infection by either AFB smear microscopy, or positive culture, or histopathology, or PCR or a combination of these (Abebe et al., 2004). 93.44% (57/61) from biopsy specimens and 68.89% (31/45) of positive cases from the curettage specimens were identified by PCR for mycobacteriums as shown in Table-II.
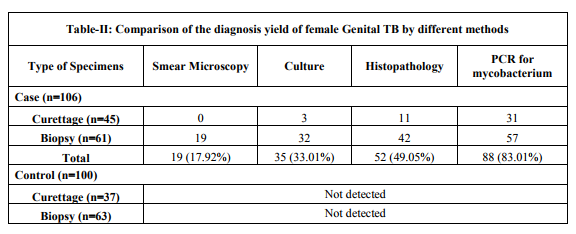
The IFN- gamma (+874) genotypes and allele frequencies of all FGTB patients and controls are shown in Table-III. Distribution of the genotypes in all groups was consistent with the Hardy-Weinberg equilibrium. The IFN-γ +874AA genotype was overrepresented in FGTB patients 19.81% when compared with the controls (9.0%) (Raut et al., 2001). Most of the FGTB patients and controls showed TA genotype (50.0% and 61.0% respectively) which is associated with intermediate IFN-γ production. However, FGTB patients also showed slight increase in frequency of TT (30.18% versus 30.0%) in comparison with controls. An increasing number of studies have shown that single nucleotide polymorphisms (SNPs) located in the promoter or coding regions of cytokine genes result in differential cytokine secretion due to altered transcriptional activation. It may be possible that different stimuli result in differential transcription of the same gene (Henao et al., 2006).
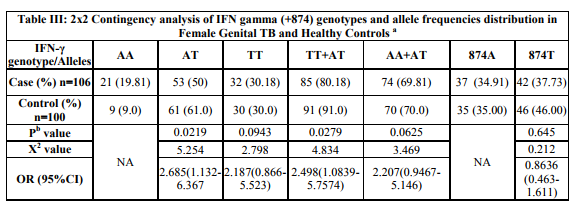
Note: a Values are given as number (percentage) unless otherwise indicated. The Pb value was evaluated by χ2 test with a 2 x 2 contingency table (genotypes) and a 2 x 2 table (allele frequencies) versus control women. b - Significance set at P ≤ 0.05. Analysis of our results showed that there was significant association in IFN-γ genotypes (AA vs. AT) between FGTB patients and control women (p value=0.0219, χ2 value=5.254, OR=2.685, 95% of CI=1.132- 6.367). i.e., individuals with IFN-γ +874 AA and (TT+AT) genotype had chi- squared value equals to 4.834, p value equals to 0.0279 and OR (95%CI) =2.498 (1.084 -5.757). No significant differences was established in IFN- γ +874AA vs. TT and AA vs. (AA+ AT) genotype frequencies between the case-control groups (p value=0.0943, X2 =2.798, OR (95%CI) =2.187 (0.866-5.523) and p value=0.0625, X2 =3.469, OR (95%CI) = 2.207 (0.946-5.146) respectively). Statistical analysis using risk of A and T alleles frequencies of FGTB patients with controls demonstrates no association with susceptibility to FGTB per se (p value=0.645). Our results are in the line with recent studies which have been reported an association between the +874 A/T SNP in the first intron of the IFN-γ gene and pulmonary TB, (Rossouw et al., 2003) suggesting that the TT genotype which is associated with lower IFN- γ production confers susceptibility to FGTB. Therefore, higher levels of IFN-γ can cause more effective cell-mediated immunity against mycobacterium. A single nucleotide polymorphism (SNP), T to A, located at position +874 in the first intron could influence IFN- γ production levels. The association of different genotypes at this position, with a low (AA), medium (AT) and high (TT) cytokine production has been shown in vitro (Lopez-Maderuelo et al., 2003). This is the first study investigating the genetic association of polymorphisms in the +874 IFN-γ gene with FGTB patients using SSPPCR.
DISCUSSION
Female genital tuberculosis is an important cause of infertility, rarely diagnosed in developed countries. It often has low-grade symptoms with very few specific complaints have been explained in the Methods and Materials. IFN-γ is required for host defense against a broad range of pathogens and is especially critical for mycobacterial immunity. Lack of production and mutations in the cytokine gene (Cooper et al., 1993) is associated with the most lethal forms of infections and increase the susceptibility to develop the disease. IFN-γ +874A allele has been previously reported to be associated with infectious diseases such as tuberculosis, hepatitis B virus infection, and parvovirus infection, (Tso et al., 2005; Ben-Ari et al., 2003) revealing its potential role in host defense against microbial infections. The mechanism by which the IFN-γ +874T/A allele influences the susceptibility to FGTB may depend on its role in the regulation of IFN-γ production. The T allele of IFN-γ +874A/T provides a binding site for the transcription nuclear factor-κB (NF-κB), which is able to regulate IFN-γ expression (Pravica et al., 2000). In particular, the +874A polymorphism in the gene for IFN-γ results in decreased IFN-γ expression (relative to the +874T variant) and has been associated with susceptibility to TB in some but not all studies (Moran et al., 2007; Vidyarani et al., 2006). Heterozygous carriers have an intermediate phenotype, suggesting that more subtle variation in the IFN-γ response pathway may underlie susceptibility to TB in outbreed human populations (Levin et al., 1995). Our observation shows that the individuals with IFN-γ +874 AA genotype is more prevalent in patients with FGTB associated clinical findings like tubal block with hydrosalphinx, tubercular salphingitis and Omental adhesions. These were not distinguished in pulmonary TB and controls as well. This nature of disease may be due to genetic variations in the bacterial strain and extraordinary virulent nature of mycobacterium. Depending upon the geographic locality and ethnicity of a population, variations have been reported in various studies regarding the occurrence and frequency of extrapulmonary tuberculosis (EPTB) in the two sexes, in different age groups and the organs involved (Kadivar et al., 2007; Sreeramareddy et al., 2008). The differences in transmissibility and virulence among M. tuberculosis strains are related to the genetic background and different lineages with specific geographical regions of the organisms (Caminero et al., 2001; Gagneux and Small, 2007). Animal infection models suggests haematogenous dissemination of infection occurs before the onset of T-cell mediated immunity (Chackerian et al., 2002) and supports the hypothesis that the ability of different strains of M. tuberculosis to produce different clinical phenotypes varies dependent upon their interaction with the host innate immune response, but the relevance of these findings to human disease remains uncertain (Manca et al., 2005). Nonetheless, it is possible that more common genetic variants such as promoter region polymorphisms that influence gene expression are associated with the disease. Therefore we suggests that, the IFN-γ +874 (A/T) alleles polymorphism were significantly associated with tuberculous bacillus infectivity and likely plays role as a genetic risk factor for the pathogenesis of FGTB in Indian women. It is also possible that low IFN-γ production may impair antimycobacterial response against FGTB infection, rendering these individuals more susceptible to Tuberculous bacillus infection other than pulmonary TB (Vidyarani et al., 2006). Although IFN-γ can overcome these phenomena in vitro, M tuberculosis can interfere with IFN-γ signaling and down regulate the transcription of IFN-γ inducible genes (Ting et al., 1999). Further, we also suggested that asymptomatic nature of the disease, accessibility of reproductive clinics, and elucidation of genes associated with virulence, pressure of susceptible factors, detection of intraspecies differences in genome sequences and gene expression studies should not be neglected during the description of FGTB.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Abebe M, Lakew M, Kidane D, Lakew Z, Kiros K and Harboe M. 2004. Female genital tuberculosis in Ethiopia. Int J Gynecol Obstet., 84:241-246.
2. Ben-Ari Z, Mor E and Papo O, et al. 2003. Cytokine gene polymorphisms in patients infected with hepatitis B virus. Am J Gastroenterol., 98(1):144-50.
3. Cahn, P., H. Perez, G. Ben, and C. Ochoa. 2003. Tuberculosis and HIV: a partnership against the most vulnerable. J. Int. Assoc. Physicians AIDS Care (Chicago) 2:106– 123.
4. Caminero J, Pena MJ and CamposHerrero MI et al. 2001. Epidemiologic evidence for the spread of a Mycobacterium tuberculosis strain of the ?Beijing‘ genotype on Gran Canaria Island. Am J Respir Crit Care Med.,164:1165–70.
5. Chackerian AA, Alt JM, Perera TV, Dascher CC and Behar SM. 2002. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun., 70: 4501–4509.
6. Chakrabarti AK, Sen S, Banerjee A and Roy K.1998. Female genital tuberculosisa retrospective study. Ind J Tub, 45:101-3.
7. Cooper MA, Dalton DK and Stewart TA. 1993. Disseminated tuberculosis in interferon γ gene-disrupted mice. J Exptl Med., 178:2243-7.
8. Crofton J, Horne N, Miller F. 1992. Clinical tuberculosis. 1 st ed. London: Macmillan Education Ltd; 502-10.
9. Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A and Stewart TA. 1993. Multiple defects of immune cell function in mice with disrupted interferongamma genes. Science, 259:1739-42.
10. Dam P, Shirazee HH, Goswami SK, Ghosh S, Ganesh A, Chaudhury K, and Chakravarty B. 2006. Role of latent genital tuberculosis in repeated IVF failure in the Indian Clinical setting. Gynecol Obstet Invest., 61(4):223-227.
11. Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA and Bloom BR. 1993. An essential role for interferon γ in resistance to Mycobacterium tuberculosis. J Exptl Med., 178:2249-54.
12. Gagneux S and Small PM. 2007. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis., 7: 328–337.
13. Giannacopoulos KCh, Hatzidaki EG, Papanicolaou NC, Relakis KJ, Kokori HG, and Giannacopoulou CC. 1998. Genital tuberculosis in a HIV infected woman: a case report. Eur J Obstet Gynecol Reprod Biol., 80:227-229.
14. Haas DW. 2000. Mycobacterial diseases. In: Mandell GL, Bennet JE, Dolin R, editors. Principles and practice of infectious diseases. Philadelphia, PA: Churchill Livingstone., 2576-607.
15. Henao MI, Montes C, Paris SC and Garcia LF. 2006. Cytokine gene polymorphisms in Colombian patients with different clinical presentations of tuberculosis. Tuberculosis., 86: 11–19
16. Hoffmann SC, Stanely EM and Cox ED, et al. 2002. Ethnicity greatly influences cytokine gene polymorphism distribution Am J Trans., 2:560-567.
17. Jindal UN, Bala Y, Sodhi S, Verma S and Jindal S. 2010. Female genital tuberculosis: early diagnosis by laparoscopy and endometrial polymerase chain reaction. Int J Tuberc Lung Dis., 14(12):1629-34.
18. Kadivar MR, Ghaneh-Shirazi R, Khavandegaran F and Karimi M. 2007. Epidemiology of tuberculosis among Afghan immigrants in Fars province, southern Islamic Republic of Iran. East Mediterr Health J., 13(4):758–64.
19. Lamba H, Byrne M, Goldin R and Jenkins C. 2002. Tuberculosis of the cervix: case presentation and a review of the literature. Sex Transm Infect., 78:62-63
20. Levin M, Newport MJ, and D‘Souza S, et al. 1995. Familial disseminated atypical mycobacterial infection in early childhood: a human mycobacterial susceptibility gene? Lancet., 345:79–83.
21. Lopez-Maderuelo, D., F. Arnalich, R and Serantes, et al. 2003. Interferon-gamma and interleukin- 10 gene polymorphisms in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med., 167:970–975
22. Maher S. G., A. L. Romero-Weaver, A. J. Scarzello, and A. M. Gamero. 2007. Interferon cellular executioner or white knight? Curr. Med. Chem., 14:1279–1289
23. Majumdar A and Satwik R. 2011. Early diagnosis of female genital tuberculosis by laparoscopy and endometrial polymerase chain reaction. Int J Tuberc Lung Dis., 15(8):1134-5.
24. Manca C, Tsenova L, Freeman S, Barczak AK and Tovey M, et al. 2005. Hypervirulent M. tuberculosis W/Beijing strains up regulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 25: 694–701
25. Miller SA, Dykes DD and Polesky HF. 1988. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research., 16(3): 1215
26. Moran A., X. Ma, R. A. Reich, and E. A. Graviss. 2007. No association between the +874T/A single nucleotide polymorphism in the IFN-gamma gene and susceptibility to TB. Int. J. Tuberc. Lung Dis., 11:113– 115.
27. Namavar Jahromi B, Parsanezhad ME and Ghane-Shirazi R. 2001. Female genital tuberculosis and infertility. Int J Gynaecol Obstet., 75:269-272.
28. Newport MJ, Huxley CM and Huston S, et al. 1996. A mutation in the interferon-gamma receptor gene and susceptibility to mycobacterial infections in man. N Engl J Med., 335:1941–9.
29. O. F. Saracoglu, T. Mungan, and F. Tanzer, 1992. Pelvic tuberculosis,? International Journal of Gynecology and Obstetrics, 37(2):115–120.
30. Pacheco AG, Cardoso CC and Moraes MO. 2008. IFNG +874T/A, IL10 21082G/A and TNF 2308G/A polymorphisms in association with tuberculosis susceptibility: a meta-analysis study. Hum Genet., 123: 477–484.
31. Parrish NM, Dick JD and Bishai WR. 1998. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol., 6:107–12.
32. Pravica V, Perrey C, Stevens A, Lee J-H and Hutchinson IV. 2000. A single nucleotide polymorphism in the first intron of the human IFN-γ gene: absolute correlation with a polymorphic CA microsatellite marker of high IFN-γ production Human Immunol., 61:863-866.
33. Punnonen R, Kiilholma P and Meurman L. 1983. Female genital tuberculosis and consequent infertility. Int J Fertil., 28:235- 238.
34. Rajamaheshwari N. 2009. Pelvic tuberculosis. Ind. J. Med. Microbio., 27(4): 361-363.
35. Richards MJ and Angus D. 1998. Possible sexual transmission of genitourinary tuberculosis. Int J Tuberc Lung Dis., 2:439.
36. Rossouw, M., H. J. Nel, G. S. Cooke, P. D. van Helden, and E. G. Hoal. 2003. Association between tuberculosis and a polymorphic NFκB binding site in the interferon gamma gene. Lancet., 361:1871–1872.
37. Sreeramareddy CT, Panduru KV, Verma SC, Joshi HS and Bates MN. 2008. Comparison of pulmonary and extrapulmonary tuberculosis in Nepal- a hospital-based retrospective study. BMC Infect Dis., 8:8 doi: 10.1186/1471-2334-8- 8.
38. Sutherland AM, Glen ES and MacFarlane JR. 1982. Transmission of genitourinary tuberculosis. Health Bull (Edinb), 40:87- 91.
39. Ting LM, Kim AC and Cattamanchi A, et al. 1999. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J Immunol., 163:3898–906
40. Tso HW, Ip WK and Chong WP, et al. 2005. Association of interferon gamma and interleukin 10 genes with tuberculosis in Hong Kong Chinese. Genes Immun., 6:358-63.
41. Uitenbroek, and Daan G. 1997. Binomial. SISA. http://www.quantitativeskills.com/sisa/dist ributions/binomial.html. (1 Jan. 2004).
42. V. S. Raut, A. A. Mahashur, and S. S. Sheth. 2001. The Mantoux test in the diagnosis of genital tuberculosis in women. International Journal of Gynecology and Obstetrics., 72 (2):165– 169.
43. Vidyarani M, Selvaraj P, Adnand AP, Jawahar MS and Adhilakshmi AR, et al. 2006. Interferon gamma (IFNγ) & interleukin-4 (IL-4) gene variants & cytokine levels of pulmonary tuberculosis. Indian J Med Res., 124: 403–410.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License