IJCRR - 4(4), February, 2012
Pages: 117-124
Print Article
Download XML Download PDF
NITRATE REDUCTASE ACTIVITY DECREASES DUE TO SALINITY IN MUNGBEAN (VIGNA RADIATA) L WILCZEK VAR. HUM_1
Author: Burhan M Padder, R M Agarwal, Zahoor A Kaloo, Seema Singh
Category: General Sciences
Abstract:Salinity can have damaging effect on plant growth either because of toxicity of specific ions or as a result of osmotic stress. Concentration of a salt has a significant role in this. Influence of two salts (NaCl and KCl) on germination and early growth of mungbean is being reported in this communication. Germination of Vigna radiata cv Hum-1 was reduced by higher concentration (100mM) of salts particularly sodium chloride. Radical length increased in treatments having lower concentrations (10mM) of sodium and potassium chloride, however, higher concentrations reduced radical length as well. All NaCl treatments and higher concentrations of potassium decreased plumule growth however plumule growth increased in 10mMKCl. Fresh and dry weight of different organs also exhibited more or less similar trends. Nitrate reductase activity reduced in sodium chloride treatments, however seedlings raised with potassium chloride treatments showed enhanced nitrate reductase activity.
Keywords: Mungbean, Nitrate reductase activity, Salinity stress, Vigna radiata.
Full Text:
INTRODUCTION
Mungbean Vigna radiata L (wilczek) is considered as one of the important legume crops characterized by a relative high content of proteins rich in leucine, phenylalanine, lysine, valine, isoleucine and certain vitamins (Oplinger et al., 1990). Mungbean is also an important pulse crop with exploited medicinal properties. Salinity is the presence of soluble salts in the soils or water which can cause stress or death to crops and vegetation, increase soil erosion, pollute drinking water and damage roads, fences, railways, buildings and natural ecosystems (Abrol, 1986). During their growth and development plants are exposed to abiotic (high and low temperature, salinity, drought, radiation etc.) and biotic (pathogen, fungus etc.) stress factors, which decrease their yield and affect the quality of their products (Habashy et al., 2008). Moreover, over 6% of the world's total land area is affected by salinity and sodicity (Munns, 2005). Most crops are salt sensitive or hypersensitive plants (glycophytes) in contrast to halophytes which are the native flora of saline environments. Some halophytes have capacity to accommodate extreme salinity because of very special anatomical and morphological adaptations or avoidance mechanisms (Flowers et al., 1986).The tolerance of salt stressed plants is dependent on their ability to accumulate inorganic solutes from external media in their tissues. Sodium chloride is one amongst the most frequently accumulated salt in soil suffering from salinity. Sahu et al., (1999) and Misra et al., (1999) found that salinity caused decrease in photosynthetic pigment content and photosystem II electron transport activity in mungbean plants. Potassium is an essential macronutrient that plays a vital role in the regulation of plant growth and development (Mengel and Kirkby, 2006; Agarwal et al. 2009). It has been reported to alleviate the severe effects of a number of biotic and abiotic stresses including drought (Umar and Bansal, 1995; Umar, 2006). Yield improvement under drought conditions may be brought about by devising management strategies, like application of potassium fertilizers, which maximize the amount of water available to the crop (Turner and Begg, 1981; Loomis 1983; Mahadhar et al., 1996). Nitrate reductase enzyme (NR) represents the rate limiting step in nitrate assimilation and can be used as a marker to estimate the capacity of plant roots and shoots to assimilate external N (Beeverz and Hazeman, 1980; Oaks, 1994). Salinity is considered to be the major factor influencing crop production in mungbean hence an attempt has been made in the present investigation to find out the general and possible salinity effects of different concentrations of KCl and NaCl on mungbean.
MATERIALS AND METHODS
Seeds of mungbean (green gram) Vigna radiata cv. Hum - 1 were taken from Rajmata Vijayaraje Scindhia Agricultural University, Gwalior. Healthy and uniform seeds were surface sterilized with 0.01% mercuric chloride solution for 2-3 minutes followed by thorough washing with distilled water, thereafter the seeds were allowed to germinate in petri plates lined with filter paper. Stress was imposed by germinating and raising the seedlings in different concentrations of NaCl and KCl solutions. Seeds treated with distilled water served as controls.
Germination percentage and growth characteristics were recorded in a routine manner. After 72 hours of sowing, the seedlings were taken and processed for the determination of activity of nitrate reductase. In vivo nitrate reductase was assayed following
Srivastava, (1974) as followed by Tiwari (1996) as outlined below: Fresh material (0.3g) was cut into small pieces and put into 5 ml tubes filled with incubation medium (0.1m phosphate buffer with 200mM KNO3 and 0.5% n-propanol v/v at 7.5 pH, maintained in dark for two hours at 30°C. Subsequently aliquot (1ml) was taken and sulphanilamide (1ml of 1% in 3NHCl) and napthethylene diamine hydrochloride (1ml of 0.02%) were added to it followed by thorough mixing. Thereafter, it was allowed to develop colour for 25 minutes and finally optical density was recorded at 540nm. Nitrate reductase activity was calculated using standard curve of nitrite and expressed in terms of µ mole nitrite produced/hr/g of the fresh weight.
RESULTS AND DISCUSSION
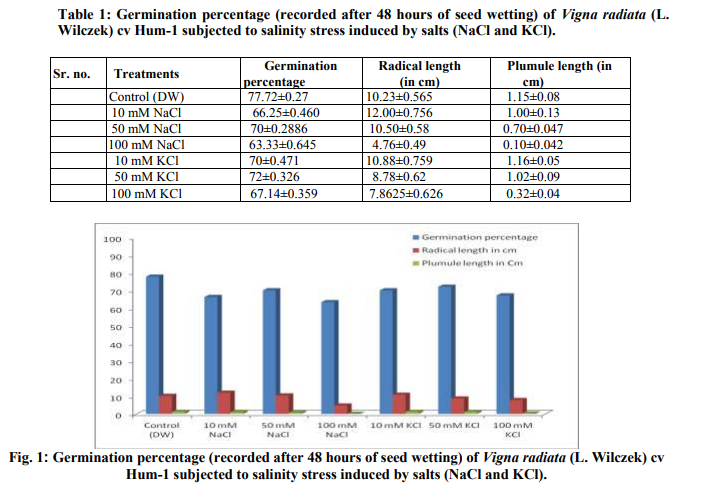
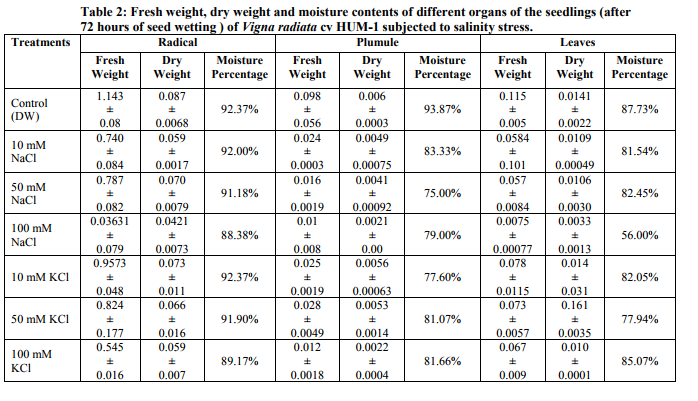
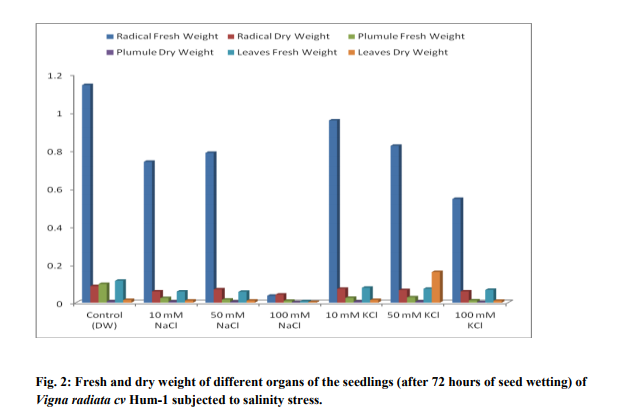
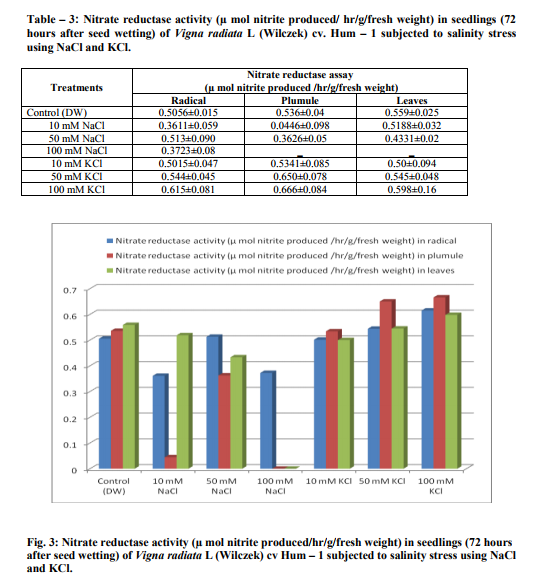
Germination of Vigna radiata cv Hum-1 was reduced by higher concentration (100mM) of salts particularly sodium chloride (63.33%) in 100mM of sodium chloride and (67.14%) in 100mM KCl as compared to (77.7%) in control. Decrease in germination percentage was in accordance with the results obtained by Misra and Diwedi (2004) in green gram (Phaselous aureus). Highest radical length was recorded with treatments having lower concentrations (10mM) of sodium and potassium chloride this could be near to optimal concentration for growth however, at higher concentration radical length was reduced. Highest plumule length was recorded in KCl (10mM). In all NaCl treatments and higher concentrations of potassium decreased plumule growth was obvious. The reduction was greater in sodium chloride treatments in case of plumule length as well (Table 1). Decrease in shoot and root length of mungbean in NaCl treatment (200mM) has also been reported earlier (Haleem and Mohammed, 2007; Akbari et al., 2008 Saffan, 2008 and Saha et al. 2010). However, we found some enhancement particularly in radical length at low concentration (10mM) of salts. The fresh and dry weight of different organs also exhibited more or less the same trend (Table 2).The results are in accordance with the findings of Musyimi et al. (2007) in Avocado seedlings grown at high salinity which resulted in large reductions in fresh and dry weight production of both shoot and root. Highest moisture percentage of radical, leaf and plumule was recorded in control and least in 100mMNaCl. Decrease in moisture percentage with increasing salinity stress is on the lines of the results reported by Meloni et al. (2004) in Prosopis alba at 600mML-1 of NaCl. Stress was comparatively greater in NaCl than KCl as in 100mMNaCl growth was strongly inhibited and so much that no sample of leaves and plumule were recovered. Increase in concentration from 10-100mMKCl resulted in increased nitrate reductase activity and the highest value observed was in plumule 100mMKCl treatment (0.666 µmol nitrite produced/ hr/g/fresh weight). Hence nitrate reductase activity was reduced in sodium chloride treatments, however, in potassium chloride treatments nitrate reductase activity showed an increase. Such observations have also been reported by Haleem and Mohammed 2007; Aslam et al., 1984, Garg et al., 1990, Dubey, 1998; Hageman and Flesher 1960; Badruddin and Dutta 2004 and Arulbalachandran 2009. Sharma and Agarwal (2002) have also reported increase in nitrate reductase activity due to potassium treatments.
The increase being greater in treatments where potassium was used as KNO3. Accompanying anions also showed an impact on the uptake of potassium as well potassium supplementation also led to depletion of nitrogen in the medium as a consequence of improved nitrogen utilization. Improved growth traits and nitrate reductase activity in potassium treatments has been reported earlier as well (Tiwari et al., 1996). However, for the present experiments we used only potassium chloride and not potassium nitrate which is reported to have greater impact on different growth parameters.

CONCLUSION
The germination of Vigna radiata cv Hum-1 was reduced by higher concentration (100mM) of salts particularly sodium chloride however, increase in radical length was recorded in treatments having lower concentration (10mM). In all NaCl and higher potassium treatments decrease in plumule growth was obvious. The reduction was greater in sodium chloride treatments in case of plumule length as well. Nitrate reductase activity was reduced in sodium chloride treatments, however, in potassium chloride treatments nitrate reductase activity showed an increase.
ACKNOWLEDGEMENTS
We are thankful to the Head School of Studies in Botany Jiwaji University Gwalior, 474011 India, for providing necessary laboratory facilities to carry out this work. Authors also acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Abrol I.P. (1986) Salt affected soils An Overview. In: Approaches for incorporating drought and salinity resistance in crop plants. (Eds.) VL Chopra and RS Paroda, oxford and IBH Publishing Co. Pvt. Ltd. New Delhi.
2. Agarwal RM, Tomar Nisha Singh, Singh Keshav and Sharma GL (2009). Potassium induced changes in flowering plants. In: Flower Retrospect and Prospect (Professsor Vishwambhar Puri Birth Centenary Volume) SR Scientific Publication, Delhi 110553 pp 158-186
3. Akbari Nasser, Barani Mohsen and Ahmadi Hadi; (2008). Effect of GA3 on Agronomic Traits of (Vigna radiata L wilczek) irrigated with different levels of saline water. World Applied Sciences Journal 5(2): 199-203
4. Arulbalachandran D, Ganesh Sankar K and Subramani A (2009). Changes in Metabolites and Antioxidant Enzyme Activity of Three Vigna Species Induced by NaCl Stress American-Eurasian Journal of Agronomy 2 (2): 109-116
5. Aslam M, Huffaker RC and Rains DW (1984). Early effects of salinity on nitrate assimilation in Barley Seedlings. Plant Physiol. 76: 321-325
6. Badruddin M and Dutta RK (2004). Nitrogen Requirement of Tomato. Indian J. Plant Physiol., 9(1): 75-78
7. Beevers L and Hageman RH (1980). Nitrate and Nitrite reduction, In: The biochemistry of Plants, Eds. Stump PK and Cou. E E. Academic Press, New York, USA, 115-168
8. Dubey RS (1998). Nitrogen metabolism in plants under salt stress (eds.) Jaiswal P K, Singh R P and Gulati A Strategies for improving Salt tolerance in higher plants 129-157 Oxford, IBH Pub. Co. Pvt. Ltd., New Delhi
9. Flowers TJ, Troke PF and Yeo AR (1977). The mechanism of salt tolerance in Halophytes. Ann. Rev. Plant Physiol., 28: 89-121
10. Flowers TJ, Hajibagheri MA and Clipson NJW (1986). In Halophytes. The Quart Rev. Biol. 61: 313-337 11. Garg BK, Kathju S, Vyas SP and Lahiri AN (1990). Effect of Saline water irrigation on tolerant and sensitive wheat varieties under desperate soil fertility conditions. Ann. Arid Zone 29: 179-189
12. Habashy NR, Zaki RN and Mahmood Awatef A (2008). Maximizing tomato yield and its quality under salinity stress in a newly reclaimed soil. Journal of Applied Sciences Research, 4(12): 1867-1875
13. Hageman RH and Flesher D (1960). Nitrate reductase activity in corn seedlings as affected by light and nitrate content of the nutrient media. Plant Physiol. 35: 700-708
14. Haleem Abdel and Mohammed MA (2007). Physiological aspects of Mungbean plant (Vigna radiata L wilczek) in response to salt stress and Gibberelllic acid treatment. Journal of Agriculture and Biological Sciences, 3(4): 200-213
15. Loomis RA (1983). Crop Manipulation for efficient use of water An overview. In: Limitations to efficient water use in crop production, 345-374
16. Mahadhar UV, Modak SI, Patil RA and Khanwilkar SA (1996). Effect of moisture regimes, nitrogen and potassium on mustard. J. Pot. Res. 12(2): 217-220
17. Melonii Diego Ariel, Gulotta Marta, Rosalia Martinez, Carlos Alberto and Olivia Marco Antonio, (2004). The effects of salts stress on growth, nitrate reduction and proline and glycinebetaine accumulation in Prosopis alba. Braz. J., Plant Physiol. 16(1) Londrina
18. Mengel K and Kirby EA (2006). Principles of Plant Nutrition 5th edition, Springer publications 439-447
19. Misra N, Dwivedi UN (1990). Nitrogen assimilation in germinating Phaselous aureus under saline stress. J. Plant physiol. 135: 719-724
20. Misra AN, Sahu SM, Misra Meena, Ramaswamy NK, Desai TS, Misra M, Horvath G and Szigeti Z, (1999). Sodium Chloride salt stress induced changes in thylakoid pigment-protein complexes, photosystem II activity and thermoluminescence glow peaks. Stress Synergisms in plants. Proceedings of an international workshop at Tata, Hungary. Zeitschrift-fur-Natrforschung. Section C, Biosciences, 54: 640-644
21. Misra, Neelam and Dwivedi UN (2004). Genotypic difference in salinity tolerance of green gram cultivars. Journal of Plant Science. 166(5): 1135-1142
22. Musyimi DM, Netondo GW and Ouma G (2007). Effects of Salinity on Growth and photosynthesis of Avocado Seedlings. International Journal of Botany 3(1): 78-84
23. Munns R (2005). Genes and salt tolerance: bringing them together. New Phytol. , 167: 645-663
24. Oaks A (1994). Primary Nitrogen assimilation in higher plants and its regulation. Can. J. Bot. 72: 739-750
25. Oplinger ES, Hardman LL, Kaminski AR, Combs SM, and Doll JD (1990). Mungbean, Alternative Field Crops Manual University of Wisconsin Extension, Cooperative Extension.
26. Saffan Samia El-Sayeed (2008). Effects of salinity and osmotic stresses on some economic plants. Journal of Agriculture and Biological Sciences, 4(2): 159-166
27. Saha K and Gupta K (1998). Effect of triazoles and CCC on growth yield and metabolism of mungbean plants under salinity stress. Indian J. Plant Physiol., 3(2): 107-111
28. Saha Papiya, Chatterjee Paramita and Biswas Ashok K (2010). NaCl pretreatment alleviates salt stress by enhancement of antioxidant defense system and osmolyte accumulation in mungbean Vigna radiata L wilczek. Indian Journal of Experimental Biology 48: 593 600
29. Sahu SM, Misra AN, Misra M, Ramaswamy NK, Desai TS and Garab G, (1998). Sodium chloride Salt Stress induced changes in thylakoid pigment protein complexes and Photosystem II activity of Vigna radiata seedlings, photosynthesis mechanisms and effects. Proceedings of the XI International congress on photosynthesis, Budapest, Hungary 4: 2625-2628
30. Sharma GL and Agarwal RM (2002). Potassium induced changes in nitrate reductase activity in Cicer arietinum L. Indian. J. Plant Physiol. 7(3): 221-226
31. Sheren Aisha, Mumtaz S, Raza S, Khan MA and Solangi S (2005). Salinity effects on seedling growth and yield components of different inbred rice lines. Pak. J. Bot. 37(1): 131-39
32. Tiwari, HS, Agarwal RM and Bhatt RK (1996). Role of Potassium on flowering and yield parameters in rice (Oryza sativa L) under normal and stressed conditions. Journal of Natural and Cultural Sciences 62(1-2): 57-58
33. Turner NC and Begg JE (1981). "Plant water relations and adaptations to stress" Plants and Soil, 58: 97-131
34. Umar S (2006). Alleviating adverse effects of water stress on yield of Sorghum, mustard and groundnut by K application. Pak J. Bot. 38: 1373-1380
35. Umar S and Bansal SK (1995). Potassium Requirement of Mustard (Brassica junca L.) under moisture stress conditions. Plants Physiol Biochem. 22(2): 130-135
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License