IJCRR - 4(6), March, 2012
Pages: 20-29
Print Article
Download XML Download PDF
A RANDOMIZED, DOUBLE BLIND STUDY TO EVALUATE THE PHARMACOLOGICAL EFFECT OF A POLYHERBAL DRUG (LIPOTAB) IN MANAGING DYSLIPIDEMIA
Author: Yasmeen Shamsi, Harendra Kumar
Category: Healthcare
Abstract:Cardiovascular disease (CVD) is the number one cause of death worldwide. Cardiovascular disease occurs usually due to atherosclerosis of large and medium sized arteries and dyslipidemia has been found to be one of the most important contributing factors. Lowering lipids through dietary intervention or pharmacological therapy has been shown to decrease the incidence of atherosclerotic events. Lipotab is a polypharmaceutical herbal drug, which has shown lipid lowering, antioxidant, anti-inflammatory and vasorelaxant activities in various animal models. To evaluate the efficacy and safety of Lipotab in dyslipidemic human subjects a randomized; double blind placebo controlled clinical study was conducted in Clinical Research Unit, Majeedia Hospital, Jamia Hamdard University, New Delhi. Twelve week Lipotab treatment, was discovered significantly effective than placebo in improving lipid profile of the study subjects. The results of the present study suggest that Lipotab is a safe and efficacious drug in treating dyslipidemia. This polypharmaceutical herbal drug can be valuable in prevention of atherosclerosis and cardiovascular disease by antiplatelet, fibrinolytic, antioxidant and
cholesterol lowering activities of its various ingredients.
Keywords: Dyslipidemia, Herbal drug, Allium sativum, Curcuma longa, Cardivascular disease
Full Text:
INTRODUCTION
Cardiovascular disease (CVD) is the number one cause of death worldwide (1, 2,). About twothirds of the estimated 14.3 million annual cardiovascular disease deaths occur in the developing world (3). CVD covers a wide array of disorders, including diseases of the cardiac muscle and of the vascular system supplying the heart, brain, and other vital organs (4). Acute coronary events (heart attacks) and cerebrovascular events (strokes) often occur suddenly, and are often fatal (5). CVD usually occurs as a result of atherosclerosis of large and medium sized arteries and dyslipidemia has been found to be one of the most important contributing factors for atherosclerosis (6). Dyslipidemia is a disorder of lipoprotein metabolism; it may manifest with the elevated levels of serum total cholesterol (TC), lowdensity lipoprotein (LDL), triglycerides, and a decrease in the high density lipoprotein (HDL) concentration (7). Atherosclerosis is usually characterized by both increased LDL-cholesterol and increased triglycerides (TG) levels and often accompanied by low HDL-cholesterol levels (8, 12). However, elevated low-density lipoprotein cholesterol (LDL) is thought to be the best indicator of atherosclerosis risk (9, 10, 11, 13). Lowering lipids through dietary intervention or pharmacological therapy has been shown to decrease the incidence of atherosclerotic events (14). A plant based diet rich in fruit, vegetables, and legumes and low in saturated fat along with regular aerobic exercise programme is an effective prescription for a person with elevated risk of cardiovascular disease (15).
Drug therapy for cholesterol reduction includes statins, bile acid resins, nicotinic acid and fibrates (15). A number of medicinal plants possess antihyperlipidemic activity, literature suggests that the lipid lowering action of herbs is mediated through, inhibition of hepatic cholesterol biosynthesis and reduction of lipid absorption in the intestine (16). These herbs may be useful in reducing the risk of cardiovascular disease. Lipotab is a polypharmaceutical herbal drug consisting of Allium sativum, Curcuma longa and Nepeta hindostan.
Pharmacological study of Lipotab and its Individual Ingredients:
The results of a study examining the endothelium modulated effects of polypharmaceutical drug Lipotab and its individual ingredients in isolated aortic rings of rat suggested a direct vasorelaxant effect of the drug on the vascular smooth muscle (17). In another study evaluating the effect of Lipotab on isoprenaline (ISO)-induced left ventricular (LV) remodeling and heart failure (HF) in Wistar albino rats, the results indicated that Lipotab prevents ISO-induced LV remodeling and consequent HF in rats through its antioxidant and anti-inflammatory activity(18) The pharmacological studies showed that supplementation with Allium sativum in cholesterol fed rabbit produced lowering in total, free, ester cholesterol and phospholipids resulting in a lower degree of atherosclerosis (19). The alcoholic extract of Nepeta hindostana (whole plant) showed cardiac stimulant activity on normal and hypodynamic heart of frog and rabbit. The alcoholic extract provided significant protection from isoproterenol-induced experimental myocardial necrosis (myocardial infarction) in rats (20). The protective effects on the cardiovascular system of Curcuma longa include lowering cholesterol and triglyceride levels, decreasing susceptibility of low density lipoprotein (LDL) to lipid peroxidation (21) and inhibiting platelet aggregation (22). Water and fat-soluble extracts of Curcuma longa and its curcumin component exhibited strong antioxidant activity, comparable to vitamins C and E (23). A study of ischemia in the feline heart demonstrated that curcumin pretreatment decreased ischemia-induced changes in the heart (24). The aim of the present study was to evaluate the efficacy and safety of Lipotab tablet in dyslipidemic human subjects.
MATERIAL AND METHODS
Study Drug:
The study drug Lipotab (500 mg) tablet is a polyherbal drug which contains dried powder of Nepeta hindostana whole plant (200 mg), Allium sativum bulb (150 mg) and Curcuma longa rhizome (150 mg). Both Lipotab and placebo were supplied by Hamdard Wakf Laboratories, New Delhi, India.
Study Design:
This was a randomized, double blind, placebo controlled study, conducted in Clinical Research Unit of Hamdard National Foundation at Majeedia Hospital, Jamia Hamdard, New Delhi, during the year 2001-2004.
Participants:
Inclusion Criteria:
Subjects (men and women) aged 25-70 years were eligible for the study if they had a history of dyslipidemia for at least 3 months despite of strict diet control and had fasting TC=200-250 mg/dl; LDL 130-170 mg/dl and TG=200-300 mg/dl. Type-2 diabetes mellitus patients with dyslipidemia were also included if they had good glycaemic control (HbA1C < 6.5%) with diet only or diet and oral hypoglycemic agents.
Exclusion criteria: Subjects were excluded from the study if they had Type 1 diabetes; uncontrolled type 2 diabetes or hypertension; hypothyroidism; nephrotic syndrome or renal failure; active hepatic dysfunction; history of coronary insufficiency/ myocardial infarction or CVD; history of estrogen therapy in post-menopausal women; women taking hormonal contraceptives, and body mass index >35 kg/m2 .
Informed Consent:
All patients were included in the study after obtaining written informed consent. Eligible subjects as per the inclusion/exclusion criteria were enrolled in the study and written informed consent was obtained from all the subjects before their enrolment. At visit 1 lipid profile determinations were conducted and the participants were instructed to follow cholesterol lowering diet for 2 weeks. After this diet only period (visit-2) lipid profile determinations and laboratory safety tests were performed and the eligible cases as per the inclusion/exclusion criteria were randomly assigned to receive either Lipotab or placebo in the dose of 2 tablets once daily at 4 p.m. All the patients were instructed to maintain low cholesterol diet as they were advised 2 weeks before their inclusion in the study (visit-1). Patients underwent an interim checkup after 6 weeks (visit-3) and a final evaluation after 12 weeks (visit-4). Physical examination and laboratory tests were done at each visit. Adverse events were recorded and compliance with study medications was assessed at visit 3 and visit 4.
Efficacy Analysis:
Data for efficacy analysis were obtained from all patients who completed 12 weeks study. Lipid profile samples were drawn from 8.30 to 9.00 Am after a 12 hour overnight fast at each visit. The change in LDL- cholesterol levels was considered the primary efficacy variable. Treatment was considered effective only of each of LDL cholesterol, total cholesterol and triglyceride levels were reduced by more than 10% compared with baseline.
Safety of the Drug: - Data from the physical examination, laboratory tests and interview for adverse events were included in the analyses of safety and tolerability. Laboratory safety tests included blood urea, serum creatinine, serum bilirubin, alanine amino transferase (AST), aspirate amino transferase (ALT), haemogram with ESR and fasting and postprandial blood sugar. All data were recorded on case record forms; analysis was restricted to patients who completed the study up to 12 weeks. The changes between pre-treatment and posttreatment values of lipid profile components obtained in drug group (Lipotab) were compared with those obtained in Placebo group by using unpaired ?t? test. Statistical calculations were performed with GraphPad InStat version 3.10.
RESULTS
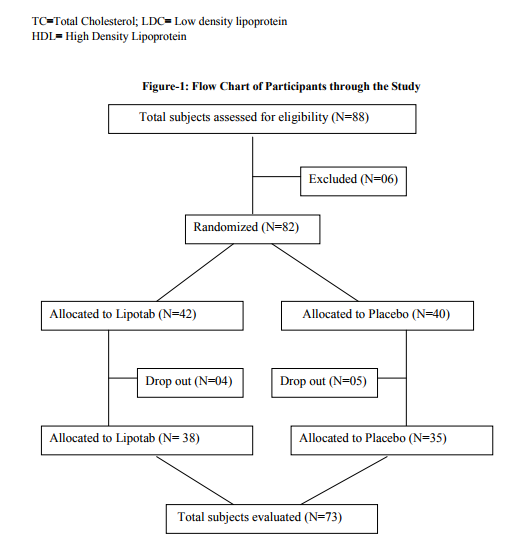
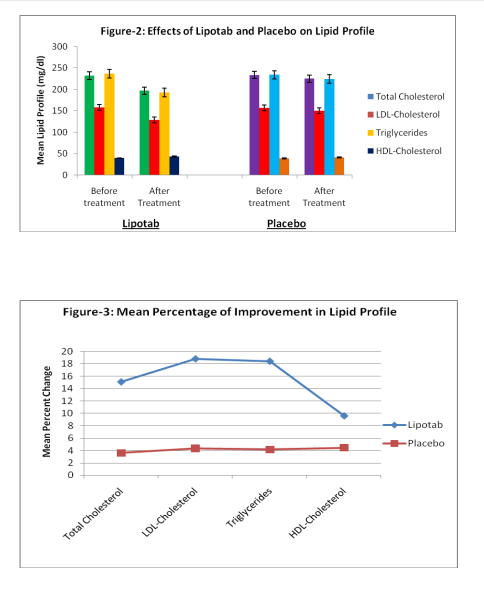
Total 88 subjects were enrolled in the study, 6 subjects did not meet the inclusion criteria because total cholesterol levels were 300 mg/dl (2 subjects), of the 82 cases included in the study, 9 cases (4 receiving Lipotab and 5 receiving Placebo) dropped out from the study for unknown/ personal reasons. Total 73 subjects (38 in Lipotab group and 35 in Placebo group) completed the study according to protocol up to 12 weeks (Figure-1) There were no significant differences in the characteristics between the two groups at baseline (Table-1 and 2). The incidence of dyslipidemia was highest in upper socioeconomic class (65.52%) and there was a moderate frequency of coronary risk factors, mainly obesity (32.18%), diabetes mellitus (29.88%), arterial hypertension (12.06%), and smoking (12.90%). The mean levels of lipid profile at baseline and after treatment and percentage of change in these levels are shown in Table-3. Twelve week Lipotab treatment, was significantly effective than placebo on the primary efficacy measure, reducing LDL-C by 18.80% compared with 4.36% in the placebo group (p < 0.001). Lipotab also significantly reduced total cholesterol (TC) by 15.09% compared with 3.625 in placebo group (<.001). Triglycerides were too reduced significantly by 18.41% with Lipotab treatment as compared to 4.16% with placebo (p<. 001). A rise in HDL –C was observed in both Lipotab (9.62%) and placebo (4.45%) group. But, this rise was significantly greater in Lipotab group as compared with placebo group (p<. 005)
Safety and tolerability: Lipotab (also placebo) treatment for 12 week did not impair physical safety indicators such as body weight, pulse rate or blood pressure. Laboratory safety indicators e.g., kidney function test (blood urea, serum creatinine), Liver function test (ALT, AST, serum bilirubin, serum alkaline phosphalase) and haemogram remained within the normal limits in all study patients. No significant change was observed in mean concentrations of blood glucose levels during the study. Lipotab was well tolerated, however 6 subjects complained of heartburn. No other adverse events like nausea, anorexia, vomiting muscle cramps and skin rash were reported
DISCUSSION
In the present clinical trial the effects of Lipotab (a polypharmaceutical herbal drug) have been observed on all the components of lipid profile in a double blind, randomized fashion and the safety of the drug has also been established. Lipotab in the daily dose of 2 tablets was discovered significantly effective in reducing mean serum LDL- cholesterol levels, which was defined in the study protocol as the main efficacy variable. As LDL-Cholesterol is the more accurate predictor of CVD. The LDL cholesterol was reduced by 18.80% with Lipotab, significant reduction in total cholesterol (15.09%), triglycerides (18.41%) was also detected with Lipotab treatment. The most important result of Lipotab treatment is the increase in HDL- cholesterol (9.62%). Allium sativum has been reported to be HMGCoA reductase inhibitor in rats (25), this supports the lipid lowering effect of Lipotab. Moreover, Lipotab has not been found associated with side effects like muscle cramps, liver dysfunction as associated with other HMG CoA- reductase inhibitors (statins) Allium sativum has also demonstrated multiple beneficial affects e.g. lowering BP, inhibiting platelet aggregation, enhancing fibrinolysing activity and protecting the elastic properties of the aorta (26). All these support the cardiovascular protective effect of Lipotab. Recent clinical trials have shown positive results for antioxidants on cholesterol levels (53, 54). Both Allium sativum (27) and Curcuma longa (23) have been reported potent antioxidants; these findings also support cardiovascular protective effect of Lipotab. Nepeta hindostana is known to prevent myocardia infarction, which supports its lipid lowering action (20). The results of the present study suggest that Lipotab is a safe and efficacious drug in treating dyslipidemia. This polypharmaceutical herbal drug can be valuable in prevention of atherosclerosis and cardiovascular disease by antiplatelet, fibrinolytic, antioxidant and cholesterol lowering activities of its various ingredients.
CONCLUSION
The results of this study can be concluded as under: Lipotab significantly reduced LDL, total cholesterol and Triglycerides as compared with Placebo Improvement in HDL obtained with Lipotab treatment was significantly greater than that of Placebo. Lipotab was well tolerated and no adverse/side effects were observed, except mild heart burn, which was reported by 6 cases.
ACKNOWLEDGEMENTS
Authors are indebted to Dr. Asad Mueed (Director) and Prof. M.S.Y. Khan (CoDirector), R and D Extension and Projects Office, CIUI, HNF, Jamia Hamdard, New Delhi, for their esteemed suggestions and kind support throughout the period of this study. We are also thankful to Dr. M.J.U. Khan, Medical Superintendent, Majeedia Hospital, for his cooperation and Administrative support during this research work. Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed
References:
1. Chaturvedi V, Bhargava B ( 2007), Health Care Delivery for Coronary Heart Disease in India- Where are we Headed. Am Heart Hosp, 5:32-37
2. Varun B. Suthar1*, Jigna S. Shah1 and Parloop A. Bhatt2 (2011), Prevalence, Assessment and Clinical Outcome Disparities in Cardiovascular Disease: IJRPC, 1(4):839
3. Mandal S, Saha JB, Mandal SC, Bhattacharya RN, Chakraborty M, Pal PP (2009), Prevalence of ischemic heart disease among urban population of Siliguri, West Bengal. Indian J Community Med. 34:19-23
4. Mathers, C. D., A. D. Lopez, and C. J. L. Murray. ?The Burden of Disease and Mortality by Condition: Data, Methods, and Results for 2001.? In Global Burden of Disease and Risk Factors, eds. A. D. Lopez, C. D. Mathers, M. Ezzati, D. T. Jamison, and C. J. L. Murray. New York: Oxford University Press.
5. World Health Organization. Prevention of Cardiovascular Disease. Guidelines for assessment and management of cardiovascular risk. Geneva, 2007
6. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), JAMA 2001;285:2486-2947
7. Chad R. Worz, Pharm.D., Michael Bottorff, Pharm.D (2003), Treating Dyslipidemic Patients with Lipid-Modifying and Combination Therapies, Pharmacotherapy ;23(5):625-637
8. Glass CK, Witztum JL (2001), Atherosclerosis: The road ahead. Cell. 104:503-516
9. Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, et al (2000) AHA Dietary guidelines: Revision 2000: A statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Circulation, 102(18):2284-2299
10. Brown M, Goldstein JL. (1984), How LDL receptors influence cholesterol and atherosclerosis. Sci Am; 251:58–66
11. Z. Chilmonczyk,D. Siluk, R. Kaliszan, B. L, Ozowicka,J. Popl⁄awski, and S. Filipek, (2001) , New chemical structures of hypolipidemic and antiplatelet activity, Pure Appl. Chem., Vol. 73, No. 9, pp. 1445–1458
12. NCEP ATP-III, 2002: Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation, 106, (25), 3143-421
13. Jacobson MS (1998), Heart healthy diets for all children: no longer controversial. J Pediatr ; 133(1):1-2
14. Byington RP, Jukema JW, Salonen JT, et al. (1995), Reduction in cardiovascular events during pravastatin therapy: pooled analysis of clinical events of the Pravastatin Atherosclerosis Intervention Program. Circulation.;92:2419–2425
15. Ashish S Phadke, (2007) A Review of Lipid Lowering Activities of Ayurvedic and Other Herbs. Natural Product Radiance, Vol. 6 (1); 81-89
16. A. Gramza, J. Korczak (2005), Trends Food Sci. Tech., 16, 351
17. Ashraf MZ, Khan MS, Hameed HA, Hussain ME, Fahim M (1999), Endothelium modulated vasorelaxant response of a polypharmaceutical herbal drug (lipotab) and its individual constituents, J Ethnopharmacol., Jul;66(1):97-102
18. Sharma A, Mediratta PK, Sharma KK, Fahim M (2011), Lipotab, a polyherbal formulation, attenuates isoprenaline-induced left ventricular remodeling and heart failure in rats, Aug; 30(8):1000-8
19. Wealth of India Vol. VII (National Institute of Science Communication Council of Scientific and Industrial Research, PID, New Delhi), 1997, 13-14
20. Satyavati GV, Gupta AK, Tondan N (1987), Medicinal Plants of India Vol.2 (Indian Council of Medical Research, New Delhi), 329-32
21. Ramirez-Tortosa MC, Mesa MD, Aguilera MC, et al. (1999), Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis,;147:371-378
22. Srivastava R, Puri V, Srimal RC, Dhawan BN (1986), Effect of curcumin on platelet aggregation Srivastava R, Puri V, Srimal RC, Dhawan BN. Effect of curcumin on platelet aggregation and vascular prostacyclin synthesis. Arzneimittelforschung, 36:715-717
23. Toda S, Miyase T, Arich H, et al. (1985), Natural antioxidants. Antioxidative compounds isolated from rhizome of Curcuma longa L. Chem Pharmacol Bull; 33:1725-1728
24. Dikshit M, Rastogi L, Shukla R, Srimal RC(1995),. Prevention of ischaemia-induced biochemical changes by curcumin and quinidine in the cat heart. Indian J Med Res 101:31-35
25. Sheela C.G., Augusti K.T (1995), Effects of S-allyl cysteine sulfoxide isolalated from Allium sativum Linn and gugulipid on some enzymes and excretions of bile acids and sterol incholesterol fed rats. Indian journal of experiments) Biology, 33/10 (749-751)
26. NickH. Mashour,GcorgeI.Liv. William,H.Frishman (1998) Herbalmedicine for the treatment of cardiovascular disease, Arch.Intern. Med., 158:2225-2234
27. Jayaprakasha GK, JenabS, Negi, PS, Sakariah KK (2002), Chemical Evaluation of antioxidant activits and antimutagencity of turmeric oil: a byproduct from curcuminproduction, Zeitschrift Fuer Naturforschung. Section C. Biosciences, 57(9-10):228-3
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License