IJCRR - 4(9), May, 2012
Pages: 148-154
Date of Publication: 17-May-2012
Print Article
Download XML Download PDF
FISH SURVIVAL: MINED OUT ACIDIC LEACHATES
Author: Vijay Yeul, Bijan Shil, Rajesh Chivane
Category: General Sciences
Abstract:This research work brings to the attention of those more concern in fish issues caused by the chemical parameters on natural resource water. To carryout research on behavior / survival rate of Puti fish in Nallah water having less content of Dissolved Oxygen and low pH due to contamination of Acid mine drainage was considered. Various concentrations of synthetic acidic leachate have been used to perform the experiment. Synthetic water is prepared in laboratory for experiment. Ranvendli nallah water is collected for preparation of synthetic water and Puti fishes are collected from same nallah. pH aned Dissolved Oxygen are essential water parameters for survival of fishes in any natural resource. Experiment conducted to study fish behavior related to the concentration of the assessed parameters, pH aned Dissolved Oxygen in Six jars. The study leads to the conclusion that though acidic leachates drop the pH aned Dissolved Oxygen of natural water but fish can survive to the great extent in the same water.
Keywords: Fish, Contaminated Water, Survival, Acidic Leachate.
Full Text:
INTRODUCTION
Once acid drainage is created, metals are released into the surrounding environment, and become readily available to biological organisms. In water, for example, when fish are exposed directly to metals and H+ ions through their gills, impaired respiration may result from chronic and acute toxicity. Fish are also exposed indirectly to metals through ingestion of contaminated sediments and food items. A common weathering product of sulfide oxidation is the formation of iron hydroxide (Fe(OH)3), a red/orange colored precipitate found in thousands of miles of streams affected by AMD. Iron hydroxides and oxy hydroxides may physically coat the surface of stream sediments and streambeds destroying habitat, diminishing availability of clean gravels used for spawning, and reducing fish food items such as benthic macro-invertebrates. Acid mine drainage, characterized by acidic metalliferrous conditions in water, is responsible for physical, chemical, and biological degradation of stream habitat. Water contaminated by AMD, often containing elevated concentrations of metals, can be toxic to aquatic organisms, leaving receiving streams devoid of most living creatures (Kimmel 1983). Receiving waters may have pH as low as 2.0 to 4.5, levels toxic to most forms of aquatic life (Hill 1974).
Data relating to specific effects of low pH on growth and reproduction (Fromm 1980) may be related to calcium metabolism and protein synthesis. Fromm (1980) suggested that a ?no effects? level of pH for successful reproduction is near 6.5, while most fish species are not affected when the pH is in a range from 5.5 to 10.5. Howells et al. (1983) reported interactions of pH, calcium, and aluminum may be important to understanding the overall effects on fish survival and productivity. Several reports indicate low pH conditions alter gill membranes or change gill mucus resulting in death due to hypoxia. Hatchery raised salmonids can tolerate pH 5.0, but below this level hemeostatic electrolyte and osmotic mechanisms become impaired (Fromm 1980). A study of the distribution of fish in Pennsylvania streams affected by acid mine drainage (Cooper and Wagner 1973) found fish severely impacted at pH 4.5 to 5.5. Ten species revealed some tolerance to the acid conditions of pH 5.5 and below; 38 species were found living in waters with pH values ranging from 5.6 to 6.4; while 68 species were found only at pH levels greater than 6.4. Further, these investigators reported complete loss of fish in 90% of streams with waters of pH 4.5 and total acidity of 15 mg/L. Healthy, unpolluted streams generally support several species and moderate abundance of 6 individuals; whereas impacted streams are dominated by fewer species and often low to moderate numbers of only a few organisms. Streams affected by acid mine drainage are poor in taxa richness and abundance. In older studies (Warner 1971), more species of insects and algae were found in unpolluted West Virginia streams (pH > 4.5) compared to those streams polluted by acid (pH 2.8 to 3.8). Reductions of benthic fauna in a West Virginia stream severely affected by acid mine water were reported by Menendez (1978). In more recent studies (Farag et al. 2003), some streams in the Boulder River watershed in Montana impacted by nearly 300 abandoned metal mines are devoid of all fish near mine sources. Populations of brook trout (Salvelinus fontinalis), rainbow trout (Oncorhynchus mykiss), and cutthroat trout (O. clarki) were found further downstream and away from sources of acid mine drainage. In a 2003 study evaluating the effect of localized habitat degradation from a gold mine near the Yukon River on population structure of salmon, it was suggested that coho salmon (O. kisutch) may be at risk of losing genetic diversity due to localized habitat degradation. The abandoned Britannia copper mine in British Columbia has been releasing acid mine drainage into local waters for many years. Investigators compared fish abundance, distribution and survival at contaminated and non-contaminated areas (Barry et al. 2000). Chum salmon (O. keta) fry abundance was significantly lower near the impacted waters (pH < 6 and dissolved copper > 1 mg/L) than the reference area. The investigators also reported that laboratory bioassays confirmed acid mine drainage from the Britannia Mine was toxic to juvenile chinook (O. tshawytscha) and chum salmon. Chinook salmon smolt transplanted to surface cages near Britannia Creek experienced 100% mortality within 2 days (Barry et al 2000). The scientific literature is replete with studies designed to quantify the adverse environmental effects of acid mine drainage on aquatic resources. Most recent investigations focus on multiple bioassessments of large watersheds.
These assessments include water and sediment chemistry, benthic macroinvertebrate sampling for taxa richness and abundance, laboratory acute water column evaluations, laboratory chronic sediment testing, caged fish within impacted streams, and development of models to explain and predict impacts of acid mine drainage on various aquatic species (Soucek et al. 2000, Woodward et al. 1997, Maret and MacCoy 2002, Hansen et al. 2002, Kaeser and Sharpe 2001, Baldigo and Lawrence 2000, Johnson et al. 1987, Griffith et al. 2004, Schmidt et al. 2002, Martin and Goldblatt 2007, Beltman et al. 1999, Hansen et al. 1999, Boudou et al. 2005). In line with the above, authors have performed an experiment on assessment of fish survival in natural nallah water, acid mined drainage contaminated nallah water and acid mined drainage effluents. In the present research work Puti fish was used for the experiment. Puti Fish ( Scientific name Swamp barb ) The swamp barb is a tropical freshwater fish belonging to the Cyprininae sub-family of the (Cyprinidae) family. It originates in inland waters in Asia, and is found in India, Pakistan, Nepal, Bangladesh, Sri Lanka, Bhutan, and Myanmar.It natively inhabits streams, rivers, canals, mangroves, marshes, swamps, ponds, and inundated fields, mainly in shallow water. They live in a tropical climate in water with a 6.0 - 6.5 pH, a water hardness of 8 - 15 dGH, and a temperature range of 68–77 °F (20– 25 °C). It feeds on worms, benthic crustaceans, insects, and plant matter.The fish will grow in length up to 6 inches (15 centimeters) and weigh up to 60 grams( http://en.wikipedia.org ) RESEARCH
METHODOLOGY AND EXPERIMENTAL
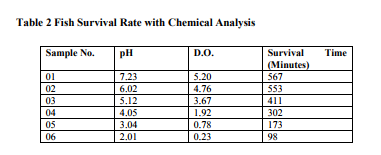
For the Puti Fish ( Scientific name Swamp barb ) species there has been made a correlation between chemical parameters viz. pH and Dissolved Oxygen of the nallah water in the jar and fish' survival rate. The experiment was done in six 2 liter jar with different chemical concentrations. One liter volume of water sample was taken in each jar. Natural Nallah Water was taken for the experiment purpose. Chemical analysis of Nallah water is given in Table No. 1. Six numbers of Puti fish recovered from same nallah from where the water sample was collected were used in experiment shown in Figure No.1 Synthetic water required for the experiment purpose was prepared by using nallah water and acidic leachate from mined out drainage of coal mines. Six Synthetic samples each of 1 liter volume were prepared from the range of pH 2 to 7 shown in Figure No.2. the concentration of water from all six jars is measured. pH was measured on calibrated table top pH meter and concentration of dissolved oxygen was measured by volumetric method.
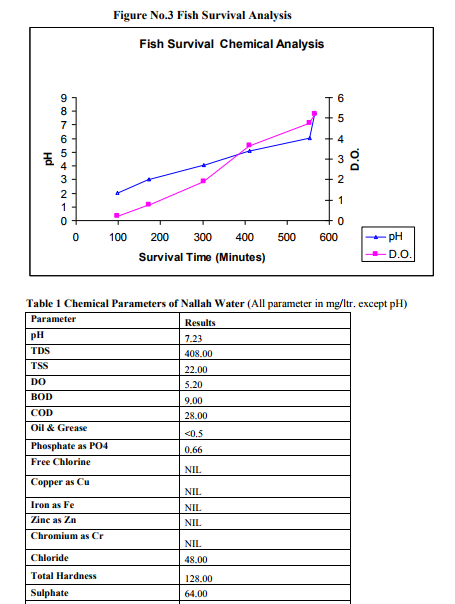
RESULTS
The results shown in Table No. 2 and Figure No.3 clearly indicate that, though the puti fish live in a tropical climate in water with a pH of 6.0 - 6.5 but from experimental results it is evidenced that it can also survive to a great extent in low pH value upto 3.04 and Dissolved Oxygen value upto 0.78 mg/liter.
DISCUSSIONS AND CONCLUSION
Acid mine drainage and associated weathering products commonly result in physical, chemical and biological impairment of surface water. Evidence from literature and field observations suggests that permitting large scale surface mining in sulfide-hosted rock with the expectation that no degradation of surface water will result due to acid generation imparts a substantial and unquantifiable risk to water quality and fisheries. It can be predicted from the above research that responses for survival of Puti fish in low oxygen and pH levels is possible and survival time in large quantity of flowing natural stream will be more than experimental condition.
ACKNOWLEDGEMENTS
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.

References:
1. Baldigo, B. P., and G. B. Lawrence (2000). "Composition of fish communities in relation to stream acidification and habitat in the Neversink River, New York." Transactions of the American Fisheries Society 129(1): 60-76.
2. Barry, K. L., J. A. Grout, C. D. Levings, B. H. Nidle, and G. E. Piercey (2000). "Impacts of acid mine drainage on juvenile salmonids in an estuary near Britannia Beach in Howe Sound British Columbia." Canadian Journal of Fisheries and Aquatic Sciences 57(10): 2031-2043.
3. Beltman, D. J., W. H. Clements, J. Lipton, and D. Cacela (1999). "Benthic invertebrate metals exposure, accumulation, and community level effects downstream from a hard rock mine site." Environmental Toxicology and Chemistry 18(2): 299-307.
4. Boudou, A., R. Maury-Brachet, M. Coquery, G. Durrieu, and D. Cossa (2005). "Synergic effect of gold mining and damming on mercury contamination in fish." Environmental Science and Technology 39(8): 2448-2454.
5. Cooper, E. L., and C. C. Wagner (1973). ?The effects of acid mine drainage on fish populations.? In: Fish and Food Organisms in Acid Waters of
6. Pennsylvania, US Environmental Protection. EPA-R#-73-032: 114. 21
7. Farag, A. M., D.Skaar, D.A. Nimick, E. MacConnell, and C. Hogstrand (2003). "Characterizing aquatic health using salmonids mortality, physiology, and biomass estimates in streams with elevated concentrations of arsenic, cadmium, copper, lead, and zinc in the Boulder River Watershed, Montana." Transaction of the American Fisheries Society 132(3): 450- 457.
8. Fromm, P. O. (1980). "A review of some physiological and toxicological responses of freshwater fish to acid stress." Environmental Biology of Fishes 5(1): 79- 93.
9. Griffith, M. B., J. M. Lazorchak, and A.T. Herlihy (2004). "Relationships among exceedances of metals criteria, the results of ambient bioassays, and community metrics in mining impacted streams." Environmental Toxicology and Chemistry 23(7): 1786- 1795.
10. Hansen, J. A., D. F. Woodward, E. E. Little, A. J. DeLonay, and H. L. Bergman (1999). "Behavioral avoidance: possible mechanism for explaining abundance and distribution of trout in a metals-impacted river." Environmental Toxicology and Chemistry 18(2): 313- 17.
11. Hansen, J. A., P. G. Welsh, J. Lipton, and D. Cacela (2002). "Effects of copper exposure on growth and survival of juvenile bull trout." Transactions of the American Fisheries Society 131(4): 690-697.
12. Hill, R. D. (1974). ?Mining impacts on trout habitat.? Proceedings of a Symposium on Trout Habitat, Research, and Management, Boone, NC, Appalachian Consortium Press. 22
13. Howells, G. D., D. J. A. Brown, K. Sadler (1983). "Effects of acidity, calcium, and aluminum on fish survival and productivity - a review." Journal of the Science of Food and Agriculture 34(6): 559-570.
14. Kimmel W. G. and Argent D. G. (1983). ?Stream Fish Community Responses to a Gradient of
15. Specific Conductance? Water Air Soil Pollut DOI 10.1007/s11270-009-0085-x,
16. Springer Science
17. Johnson, D. W., H. A. Simonin, J. R. Colquhoun, and F. M. Flack (1987). "In situ toxicity tests of fishes in acid waters." Biogeochemistry 3(1-3): 181-208.
18. Kaeser, A. J., and W. E. Sharpe (2001). "The influence of acidic runoff episodes on slimy sculpin reproduction in Stone Run." Transactions of the American Fisheries Society
19. Maret, T. R., and D. E. MacCoy (2002). "Fish assemblages and environmental variables associated with hard-rock mining in the Coeur d‘Alene River Basin, Idaho." Transactions of the American Fisheries Society 131(5): 865-884.
20. Martin, A. J., and R. Goldblatt (2007). "Speciation, behavior, and bioavailability of copper downstream of a mine-impacted lake." Environmental Toxicology and Chemistry 26(12): 2594-2603.
21. Menendez, R. (1978). ?Effects of acid water on Shavers Fork – a case history.? Surface mining and fish/wildlife needs in the Eastern United States., U.S. DOI, Fish and Wildlife Service. FWS/OBS 78/81: 160-169.
22. Schmidt, T. S., D. J. Soucek, and D. S. Cherry (2002). "Modification of an ecotoxicological rating to bioassess small acid mine drainage-impacted watersheds exclusive of benthic macroinvertebrate analysis." Environmental Toxicology and Chemistry 21(5): 1091-1097.
23. Soucek, D. J., D. S. Cherry, R. J. Currie, H. A. Latimer, and G. C. Trent (2000). "Laboratory and field validation in an integrative assessment of an acid mine drainage-impacted watershed." Environmental Toxicology and Chemistry 19(4): 1036-1043.
24. Warner, R. W. (1971). "Distribution of biota in a stream polluted by acid mine drainage." Ohio Journal of Science 71(4): 202-215.
25. Woodward, D. F., J. K. Goldstein, A. M. Farag, and W. G. Brunbaugh (1997). "Cutthroat trout avoidance of metals and conditions characteristic of a mining waste site: Coeur d‘Alene River, Idaho." Transactions of the American Fisheries Society 126(4): 699-706.
26. http://en.wikipedia.org/swampbarb
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License