IJCRR - 4(9), May, 2012
Pages: 129-142
Date of Publication: 17-May-2012
Print Article
Download XML Download PDF
A RANDOMIZED, DOUBLE BLIND STUDY OF THE SAFETY AND EFFECTIVENESS OF A POLYHERBAL DRUG IN THE TREATMENT OF ACTIVE RHEUMATOID ARTHRITIS
Author: Yasmeen Shamsi, Harendra Kumar
Category: Healthcare
Abstract:Introduction: Rheumatoid arthritis (RA) is a common disease characterized by chronic inflammation and erosion of the joints associated with functional impairment. The main therapy for symptomatic relief in RA is based on NSAIDs as the first group of drugs utilized all over the world; however they are frequently associated with a fair degree of side effects. Methods: We conducted a randomized double blind, placebo controlled trial to assess the efficacy and safety of a polyherbal drug Qurs-e-Mafasil in patients of active rheumatoid arthritis. Total 125 patients completed the study up to 12 weeks; they received either Qurs-e-Mafasil or Placebo in the dose of two tablets thrice daily. Efficacy and safety assessment was done at base line and then at every 4-week's treatment up to 12 weeks. Results: In Qurs-e-Mafasil treatment group marked improvement was achieved in tender joint count (p =0. 002), swollen joint count (P=0.001) and pain intensity score (P=0.0001). This improvement was significantly greater as compared to Placebo group. There was no significant difference between treatment groups regarding the number of patients that reached ACR20 improvement criteria in the assessments after 12 weeks of treatment, but improvement in quality of life is significantly greater in Qurs-e-Mafasil group than in placebo group. Conclusion: Qurs-e- Mafasil exerted significant effect in pain relief, reduction in tender and swollen Join count and functional improvement without producing adverse effects.
Keywords: Rheumatoid Arthritis, Unani Drug, Colchicum luteum, Curcuma longa
Full Text:
INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune disease that causes chronic inflammation and erosion of the joints (1). It is a progressive disease associated with severe morbidity, functional impairment, permanent disability and increased mortality 2, 3, 4, 5. About 1% of the world's population is distressed by rheumatoid arthritis, women three times more often than men. Onset is most frequent between the ages of 40 and 50, but people of any age can be affected6, 7, 8. The main therapy in RA is based on NSAIDs (Nonsteroidal Anti-Inflammatory Drugs) as the first group of drugs utilized all over the world 9, 10. The NSAIDs are efficacious and very helpful in relieving pain, but they are frequently associated with a number of side effects, some of which are significant and potential life threatening including gastrointestinal symptoms, alteration of platelet function and impairment of renal function 9 . Herbal remedies have been popular since antiquity of being relatively less expensive and free of adverse effects. For the treatment of arthritis and joint pain a number of herbal drugs have been mentioned in classical Greco-Arabic medical literature. The study drug ?Qurs-eMafasil? is a polyherbal Unani formulation; in clinical practice this drug has been found effective and safe in the treatment of joint pain and inflammation. But, this drug has not been substantiated in well-controlled clinical trials. Hence, a randomized, double blind, placebo controlled study was planned to evaluate the efficacy and safety of Qurs-e-Mafasil in the treatment of patients with rheumatoid arthritis.
METHODS
We conducted this randomized, double blind, placebo controlled study at Hamdard National Foundation‘s Clinical Research Unit, Majeedia Hospital, Jamia Hamdard, New Delhi, India, from September 2003 to March 2007.
Selection of Patients:
Inclusion Criteria: Patients of 20-70 years of age fulfilling the criteria of American College of Rheumatology (ACR) for the diagnosis of rheumatoid arthritis18,19 who had never received disease modifying anti rheumatic drugs (DMARDs) were included in the study. The other criteria for entry were the presence of active disease as defined by at least three of the following criteria: six or more swollen joints, six or more tender joints, 45 minutes or more of morning stiffness, and an erythrocyte sedimentation rate (Westergren) exceeding 28 mm an hour.
Exclusion Criteria:
- Patients were excluded if they had one or more of the following criteria:
- Presence of deformities
- Advanced radiographic changes in any joint Concomitant renal/ hepatic insufficiency or sever gastrointestinal insufficiency
- Pregnant and lactating women Prior treatment with DMARDs/corticosteroids
Study Drug:
The study drug ?Qurs-e-Mafasil? (in the form of tablet) is a polyherbal Unani formulation, each Qurs-e- Mafasil tablet (500 mg) contains fine powder of five herbs namely Colchicum luteum (100 mg), Commiphora mukul (100 mg), Curcuma longa (100 mg), Terminalia chebula (100 mg) and Delphinium denudatum (100 mg). In classical Greeko-Arabic medical literature these herbs have been described to be effective in the treatment of various kinds of arthritis 11- 17 . Both Qurs-e-Mafasil and Placebo were supplied by Hamdard (Wakf) Laboratories, New Delhi. Dosage and Administration:
Following 5-7 days washout period of antiinflammatory or analgesic drugs (e.g., NSAIDs, Corticosteroids) or any other medication used for the treatment of arthritis (e.g. Ayurvedic, Homeopathic or Unani drugs), patients were randomly assigned to receive either Qurs-eMafasil or placebo treatment. Randomization was achieved by the generation of a randomization list using permutable blocks. Both Qurs-e-Mafasil and placebo (Weight of each tablet= 500 mg) were given in the dose of two tablets thrice daily after meals. Duration of
Treatment: The study was continued up to six weeks as a double blind placebo controlled study. Later on the study was continued as an open label trial up to 12 weeks.
Concomitant Treatments:
No concomitant treatment was allowed during the study. The patients who were already taking any anti-inflammatory or analgesic drugs were advised to stop all these medications 5-7 days prior to the administration of study medication.
Ethical Consideration:
The study was approved by Institutional Ethics Committee (IEC) of Jamia Hamdard, New Delhi for conducting clinical trials of herbal drugs on human beings, and registered in WHO- ICMR Clinical Trial Registry vide CTRI No. CTRI/2009/000746 (www.ctri.nic.in) All patients were included in the study after obtaining written informed consent.
Efficacy Measures and Follow-up: Primary Outcome: The primary outcome measures included reduction in the followings:
- Swollen Joint count
- Tender Joint Count
- Intensity of pain- VAS (0-10)
- Duration of Morning Stiffness (Minutes)
- Patient‘s Global Assessment of disease activity (0-10)
- Physician‘s Global Assessment of disease activity (0-10)
- ESR (mm/1hour)
Secondary Outcome: The secondary outcome was the proportion of patients that reached clinical response of at least 20%, as defined by the ACR20 criteria.20 The secondary outcome also included improvement in quality of life as assessed by Health assessment Questionnaire (HAQ) disability index.21 Clinical Assessment: Clinical evaluation was done at base line (0 week) and then at every 4-week's treatment up to 12 weeks. The disease activity was measured by: i) Counting tender and swollen joints ii) Patient's evaluation of pain (Visual Analogue Scale- VAS (0-100). iii) Duration of early morning stiffness (in minutes) The functional disability was measured by Health Assessment Questionnaire (HAQ) of Stanford University School of Medicine (Division of Immunology and Rheumatology) 21 .
Data Analysis:
All data were recorded on case record forms; analysis was restricted to patients who completed the study up to 12 weeks. The changes between pre-treatment and posttreatment mean scores of disease activity measures obtained in drug group (Qurs-eMafasil) were compared with those obtained in Placebo group by using Mann Whitney ?U‘ test. Level of significance was set at 5% and twosided P-values are given. The baseline characteristics of the two groups were also compared by applying Mann Whitney ?U‘ test. Pair wise (within group) comparisons for each group independently were based on the Wilcoxon matched pairs signed-ranks test. A p value <0.05 was considered significant Statistical calculations were performed with GraphPad InStat version 3.10.
Safety Evaluation:
At each visit, the patients were asked about the adverse reactions without suggesting that these should be expected. Additionally, haemogram (Hb%, TLC, DLC, Platelet Count, ESR), liver function tests (SGOT, SGPT, Serum Alkaline Phosphatase, Serum Bilirubin) and kidney function tests (Blood Urea, Serum Creatinine) were performed to assess the safety of the drug.
Treatment compliance
Treatment compliance of 100% was obtained if a patient took 42 tablets within a period of 7 days. The percentage of treatment compliance was defined as: Compliance (%) =Total number of tablets taken/42 x 100
RESULTS
Sample Characteristics
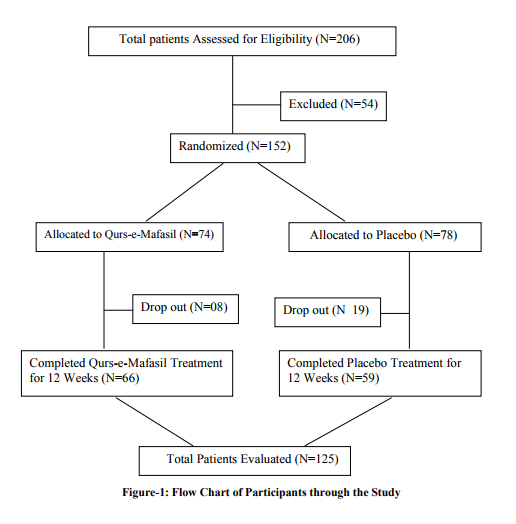
Total 206 patients of active rheumatoid arthritis were screened to enter the study, out of them 152 met the eligibility criteria. Of these 152 patients, 74 were randomly assigned to receive Qurs-e-Mafasil and 78 were assigned to receive placebo. Forty-seven patients (08 in the Qurs-e-Mafasil treatment group and 19 in the placebo group) did not complete the study up to 12 weeks. The reason for drop out in both the groups was either unspecified or lack of treatment effect. Most of them dropped out before the end of the first study month. Total 125 patients (66 in Qurs-e-Mafasil group and 59 in placebo group) completed the study according to protocol up to 12 weeks (Figure-1)
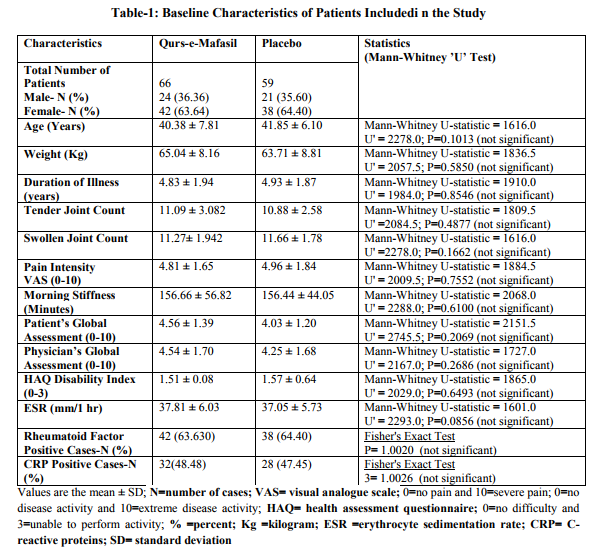
Baseline Characteristics
The patients treated with Qurs-e-Mafasil (N= 66) and the placebo-exposed patients (N= 59) were comparable at baseline. The demographic and baseline clinical features for patients in the two treatment groups are shown in Table1. The statistics comparing demographic variables and baseline disease characteristics is also expressed in Table 1, which showed that there was no significant difference between the Qurs-e-Mafasil and placebo groups for initial distribution of any of the studied parameters.
Clinical Efficacy:
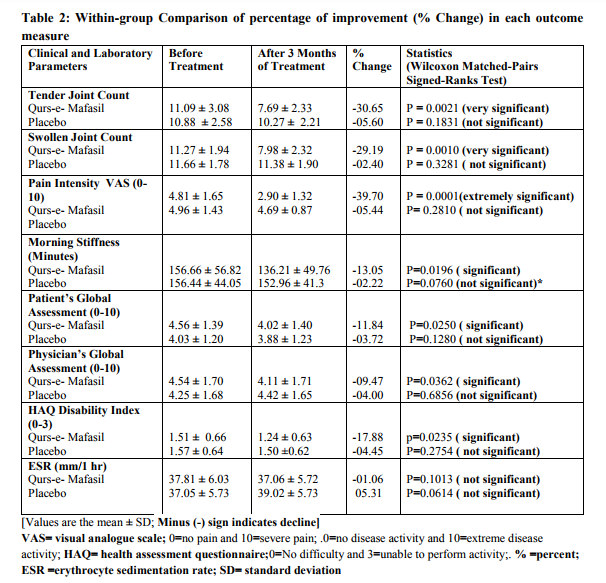
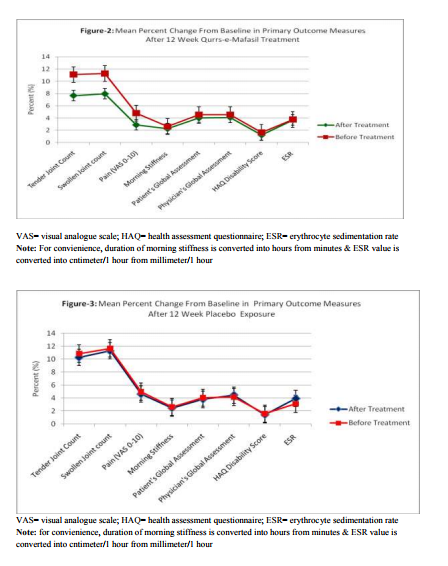
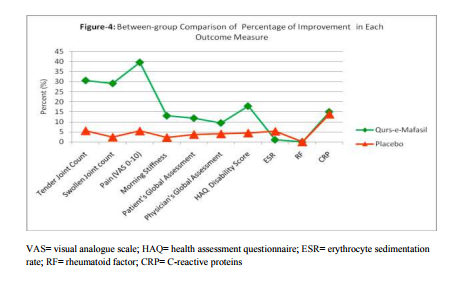
Within-group Comparison: Table 2 and Figures 2 and 3 illustrate the changes from baseline in tender joint count, swollen joint count, VAS for pain, morning stiffness, Patient‘s Global Assessment, Physician‘s Global Assessment and Functional Disability Index (HAQ) score, and ESR. After 12 weeks of Qurs-e-Mafasil treatment, marked improvement was observed in tender joint count (30.65%; p=0.0021 vs placebo= 5.60%; p=0.1831), swollen joint count (29.19%; p=0.0010 vs placebo= 2.40%; p= 0.3281) and pain intensity score (39.70%; p=0.0001 vs placebo=5.44; p=0.281). Qurs-eMafasil treatment produced modest improvement in morning stiffness (13.05% p=0.0196 vs placebo=02.22%; p=0.0760), patient‘s global assessment of disease activity (11.84%; p=0.0250 vs placebo= 03.72%; p=0.1280) and physician‘s global assessment of disease activity (09.47%; p=0.0360 vs placebo= 04.00%, P=0.6856).Significant improvement from baseline in HAQ disability scores was also found with Qurs-e-Mafasil treatment (17.88%; p= 0.0235 vs placebo=04.45%; p= 0.2754). Mild improvement in ESR titre was noticed in some Qurs-e-Mafasil treated patients, but the improvement in mean ESR did not reach statistical significance (01.06%; p=0.1013). Conversely, in the Placebo group the mean ESR titre was significantly raised from baseline (05.31%; p=0.0414). Clinical Efficacy: Between Group Comparison of Primary It was determined on statistical analysis (MannWhitney test, as expressed in Table-3 and Figure4 that there were significant differences in most of the primary outcome measures at week 12 between the two groups. The improvement in joint pain, tender joint count and swollen joint count was markedly greater in Qurs-e-Mafasil group as compared to placebo group. On statistical analysis extremely significant differences were detected in the improvements of these three components between the two treatment groups (p=0.0001 % in all three constraints) as evident from Table-3 and Figure4. The mean percent changes in rheumatoid factor and CRP positive cases in both the groups were not be different significantly as analysed by applying Fisher's Exact Test (Table-3 and Figures-4). Between Group Comparison of Secondary Outcome: An ACR20 response at week 12 was achieved in 5 of 66 patients (7.57%) in the Qurs-eMafasil group compared with 3 of 59 patients (3.38%) in the Placebo group (p=0.021). The improvement in quality of life of patients was significantly greater in Qurs-e-Mafasil group than that of Placebo group, as made clear in Table-3 and Figure-4. Treatment compliance: On the basis of the number of days in the study (90 days) and the number of returned tablets, the calculated compliance was 96% in the Qurse-Mafsil group and 93% in the Placebo group. Safety and Tolerability Overall, both Qurs-e-Mafasil and Placebo were well tolerated. However, three out of 66 patients complained mild gastrointestinal upset in the initial 4-5 days of Qurs-e-Mafasil treatment and then relieved spontaneously.None of the adverse events led to study discontinuation and there were no serious adverse events during the study. There were no clinically relevant changes from baseline in vital signs, physical findings and other safety parameters e.g. Hb%, TLC, LFT and KFT in either group during the study.
CONCLUSION
The results of the present study can be concluded as under: ? Qurs-e-Mafasil provided clinically meaningful improvements in pain, swelling and tenderness of joints that were superior to those of placebo. ? Fair improvements were achieved in morning stiffness, patient‘s and physician‘s global assessment of disease activity with Qurs-eMafasil treatment, which were superior to those of placebo. ? The quality of life of patients significantly improved with Qurs-e-Mafasil treatment, whereas no improvement in quality of life was observed in placebo exposed patient ? Qurs-e-Mafasil failed to decrease the raised ESR levels; conversely a significant rise in ESR levels was noticed in placebo group. ? Qurs-e-Mafasil and placebo too did not produce any improvement in rheumatoid factor and Creactive proteins. ? There was no difference between the Qurs-eMafasil and the placebo groups in the number of patients that reached the ACR 20 improvement criteria. Moreover, ACR 20 response was achieved in a fairly small number of patients in both the groups (5 of 66 Qurs-eMafasil treated patients and 3 of 59 Placebo exposed patients). ? Qurs-e-Mafasil was discovered safe and well tolerated; no serious adverse events were reported by any of the patients. However, three out of 66 patients complained mild gastrointestinal upset in the initial 4-5 days of treatment and then relieved spontaneously.
DISCUSSION
The results of this placebo controlled, double blind randomized study proved that Qurs-eMafasil produced strong effect in reducing joint pain, swollen and tender join counts, consequently the quality of life of patients in this group improved quite significantly. Fair improvements were also observed in morning stiffness and patient‘s and physician‘s global assessment of disease activity.in Qurs-e-Mafasil treated group of patients. Conversely, in placebo group no statistically significant improvements were found in all these constraints. No significant change in laboratory parameters (ESR, Rheumatoid Factor and CRP) was found in both the treatment groups; however the Qurs-eMafasil group showed a little improvement in haemoglobin percentage, though this improvement was not found significant when analysed statistically. Conversely, the placebo group showed a little non-significant reduction of haemoglobin and significant rise of the ESR. During the whole study period no serious adverse effects were reported in either intervention group except mild gastrointestinal upset (Nausea, epigastric discomfort, diarrhoea), which were reported in 3 out of 66 patients treated with Qurs-e-Mafasil. In summary, Qurs-e-Mafasil in the dose of 2 tablets thrice daily provided clinically meaningful improvements in most of the disease activity constraints and quality of life without any significant adverse effect in patients with active rheumatoid arthritis. A limitation of the present study is the absence of radiographic evaluation. The primary analysis was focused on clinical signs and symptoms rather than on joint damage measured by radiography; we therefore do not know whether a more robust benefit than shown in the clinical findings would have been achieved if radiographs had been evaluated. The aim of current rheumatoid arthritis (RA) treatment is to control disease activity, alleviate symptoms, maintain physical function, optimize quality of life, and slow the rate of joint damage. The Pharmacotherapy for rheumatoid arthritis generally involves a nonsteroidal antiinflammatory drug (NSAID) for control of pain, with a disease modifying antirheumatic drug (DMARD). The NSAIDs are efficacious and very helpful in relieving pain, but they are frequently associated with a number of side effects, some of which are significant and potential life threatening including gastrointestinal symptoms, alteration of platelet function and impairment of renal function7 . On the basis of the results of this study, it is put forward that Qurs-e-Mafasil is a safe drug and may be used as an anti-inflammatory agent to relieve joint pain, swelling and tenderness. Furthermore, it may be used with DMARDs in place of NSAIDs, but further extensive studies in large number of patients with radiological assessment are warranted.
ACKNOWLEDGEMENTS
We are indebted to Dr. Asad Mueed (Director) and Prof. M.S.Y. Khan (Co-Director), R and D Extension and Projects Office, CIUI, HNF, Jamia Hamdard, New Delhi, for their esteemed suggestions and kind support throughout the period of this study. We are also thankful to Dr. M.J.U. Khan, Medical Superintendent, Majeedia Hospital, for his cooperation and Administrative support during this research work. Authors acknowledge the immense help received from the scholars whose articles are cited and included in reference of this manuscript. The authors are also grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Lina M Olsson, Anna-Karin Lindkvist, Henric Kallberg, Leonid Padyukov, Harald Burkhardt, Lars Alfredsson, Lars Kalareskog and Rikard Holmdhal, A Case Control Study of Rheumatoid Arthritis, Identifies an Associated Single Nucleotide Polymorphism in the NCF4 Gene, Supporting a Role for the NADPH-Oxidase Complex in Autoimmunity, Arthritis Research and Therapy, 2007; 1-17
2. Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, VaughnWK Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum, 1984; 27(8):864–872
3. Brosseau L, Welch V, Wells G, deBie R, Gam A, Harman K, Morin M, Shea B and Tugwell P, Low Level Laser Therapy (Classes I,II and III) in the Treatment of Rheumatoid Arthritis, Cochrae Database Syst. Review, 2000; (2): CD002049
4. Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK, Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum, 1984; 27(8):864–872
5. Pincus T, Brooks RH, Callahan LF, Prediction of long-term mortality in patients with rheumatoid arthritis according to simple questionnaire and joint count measures. Ann Intern Med.1994; 120 (1):26–28
6. Brosseau L, Welch V, Wells G, deBie R, Gam A, Harman K, Morin M, Shea B and Tugwell P, Low Level Laser Therapy (Classes I,II and III) in the Treatment of Rheumatoid Arthritis, Cochrae Database Syst. Review, 2000; (2): CD002049.
7. Bardwell W A, Nieassio P M, Weisman, M H, Gevirtz R, and Bazzo D, Rheumatoid Arthritis Severity Scale: A Brief, PhysicianCompleted Scale Not Confounded by Patient Self Report of Psychoilogical Functioning, Rheumatology, 2002; 41:38-45.
8. Kari Puolakka, Hannu Kotiainen, Timo Mottonin, Pekka Hannonen, Markku Korpela, Heikki Julkunen, Reijo Luukkainen, Kaisa Vuori, Leena Paimela, Harri Blafield, Markku Hakata and Marjatta Leirisalo-Repo, Impact of Initial Aggressive Drug Treatment with a Combination Disease-Modifying Anti rheumatic Drugs on the Development of work Disability in Early Rheumatoid Arthritis, Arthritis and Rheumatism, 2004; 50 (1), 55-62
9. Majithia V, Geraci SA, "Rheumatoid arthritis: diagnosis and management". Am. J. Med., 2007; 120 (11): 936–9
10. Sean Z. Zaho, Justus I. Fiechtner, Elizabeth A. Tindall, Seema D. Dedhiya, William W. Zhao, Jane T. Osterhaus and Shawn S.Yu, Evaluation of Health Related Quality of Life of Rheumatoid Arthritis Patients Treated with Celecoxib, Arthritis Care and Research, 2000; 13(2), 112-121.
11. Nair V, Singh S, Gupta YK, Evaluation of the disease modifying activity of Colchicum luteum Baker in experimental arthritis, J Ethnopharmacol. 2011 Jan 27;133(2):303-7.
12. Unani Pharmacopoeia Committee. Zard Chob. In: The Unani Pharmacopoeia of India, Part I, Volume I. New Delhi, India: Government of India, Ministry of Health and Family Welfare, Department of Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homoeopathy (AYUSH). 2007;90-91. (Haldi)
13. Satyavati, G.V. Guggulipid: a promising hypolipidemic agent from guggul (Commiphora wightii). Economic and medicinal plant Research, Plants Traditional Med.1991;, 5: 47 136 International Journal of Current Research and Review www.ijcrr.com Vol. 04 issue 09 May 2012
14. Tripathi, R.M., Gupta, S.S. and Chandra, D.,. Antitrypsin and Anti-hyaluronidase activity of Curcuma longa (Haldi). Indian J. Pharmac, 1973; 5: 260-261
15. Narasimhan, S., Anitha, G., Illango, K. and Mohan Kumar, R.,. Bio-assay guided isolation of active principles from medicinally important plants. In: Herbal Drugs: A twenty First century Perspective. (Sharma, R.K. and Arora, R. Eds). JAYPEE Brothers, New Delhi , 2006;pp- 70-76.
16. Rastogi and Mehrotra, Compend. India Med. Plants,PID, New Delhi, 1990;1:406-408
17. Nizami, Qudsia and M.A. Jafri, Unani drug, jadwar (Delphinium denudatum Wall.) -- a review, Indian Journal of Traditional Knowledge, 2006; 5 (4), 463—467
18. Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology: preliminary definition of improvement in rheumatoid arthritis. Arthritis Rhe Rheum 1995;38:727–35
19. Akil M, Amos RS. ABC of rheumatology. Rheumatoid arthritis-I: clinical features and diagnosis. BMJ 1995; 310:587-90.
20. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31:315-24
21. Khan Mohammad Azam, Ikseer –e-Azam, Urdu Translation by Hkm. Mohammad Kabiruddin, Tibbi Company, Rawalpindi, Pakistan, 1939, Vol.II, pp. 1430-48

|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License