IJCRR - 4(11), June, 2012
Pages: 145-150
Date of Publication: 18-Jun-2012
Print Article
Download XML Download PDF
IN-VITRO STUDIES OF VITEX NEGUNDO L. AN IMPORTANT MEDICINAL PLANT
Author: Firdous Dar, Kirti Jain, Madhuri Modak
Category: General Sciences
Abstract:A rapid and efficient protocol was developed for shoot induction and multiple shoot formation from apical and nodal explants of Vitex negundo L. an important endangered medicinal plant species. The explants were cultured on Murashige and Skoogs (MS) medium supplemented with various concentrations of auxins, cytokinins and sucrose. Highest percentage (97%) of shoot induction were observed from axillary meristem, developed 10-20 shoots when cultured in the medium containing the combination of 6 -Benzyl amino purine (BAP) (0.5 mg/l) and Naphthalene acetic acid (NAA) (0.2mg/l) supplemented with 3% Sucrose within 25 days.
Keywords: In-vitro studies, Benzyl amino purine, Naphthalene acetic acid, Vitex negundo L.
Full Text:
INTRODUCTION
Verbenaceae is a large family of herbs, shrubs and trees comprising of about 75 genera and nearly 2500 species (Nasir and Ali 1974., Sastri 1950). V. negundo L. is distributed in East Asia, South West China, throughout the greater part of India at warmer zones and ascending to an altitude of about 1500m in outer, western Himalayas. It is also cultivated in Pakistan. (Usman Ghani Khan, 2007., Khare, 2007., Cook, 1903). V. negundo L. is a large woody aromatic and multipurpose medicinal shrub belonging to the family verbinaceae (Wealth of India 1976). It is one of the common plants used in Indian system of medicine. Various parts of the plant are used in the treatment of Arthritis, joint pains and sciatica. It is also used in the treatment of chronic bronchitis, asthma and gastric troubles. In dispersing swellings of the joints from acute rheumatism and also of the testes from suppressed gonorrhea. The methanolic root extracts of V.negundo significantly antagonized the Vipera russellii and Naja kauthea venom induced lethal activity both in-vitro and in-vivo studies (Alam and Gomes, 2003). The stem decoction is used in the treatment of burns and scalds. The fresh berries are pounded to a pulp and are used in the form of a tincture for the relief of paralysis, pains in the limbs, weakness etc. The leaves of the plant are astringent, febrifuge, sedative tonic and vermifuge (Horowitz, 1996). The plant also shows antibacterial, antifungal, larvicidal, antihelmentic, antioxidant, and insectsidal/pestcidal activities. The plant also shows anticancerous activity against Daltons Asiatic lymphoma. It also shows gastro protective and hepato-protective activities. Despite its economic importance the production of V.negundo is threatened by population growth, desertification, industrial development and attack by numerous parasites. The biotechnological approach such as plant tissue culture initiated from medicinal plants is a variable method for the large scale production of economically and medicinally important plants. The present study was undertaken to standardize a protocol for high frequency induction of multiple shoots from different explants and to regenerate plants of V.negundo to meet its demand in medicine and agriculture.
MATERIALS AND METHODS
Actively growing and healthy shoot material of V.negundo with dominant auxiliary buds were collected from an adult plant growing in the medicinal plant garden of Govt. Motilal Vigyan Mahavidyalaya Bhopal, M.P. After removing leaves, the shoots were cut into small pieces 0.5- 1.0 cm each containing a single node auxiliary bud. The explants were then washed under running tap water for 30 minutes, followed by a wash with a solution of detergent for 10 min. followed by washing with surface sterilizing agent mercuric chloride (0.1%HgCl2) solution for 3-6 min. In sterilized autoclaved beakers and finally washed three times with autoclaved water. Since the use of sodium hypo chloride and bromine water did not prevent contamination. Mercuric chloride was used as sterilizing agent throughout the experiment. The explants were then inoculated in basal medium consisting of Murashige and Skoogs salts, vitamins 30g/L. Sucrose 30g/L. Agar (qualigens India) supplemented with various growth hormones. After adjusting the PH (5.4-5.9) the medium was autoclaved at 121oC for 15-20 minutes at the pressure of 1.06 kgcm-2 . The cultures were then incubated at 25+3 oC under 14/10 hours (light/dark) period with light supplied by white fluorescent tubes at 3500 lux. After 20 days of inoculation, the explants were transferred to a fresh medium. And after 40 days of inoculation data were recorded on shoot induction and number of shoot formation per explant. For multiplication of cultures in-vitro raised shoots were taken in a sterilized Petri dish and were cut into small pieces containing a single node along with dormant auxiliary buds. Then the explants were transferred to culture tubes containing MS medium supplemented with BAP (2mg/L.) and NAA (0.5mg/L.). For the induction of multiple shoots, subsequently subcultures were raised after 20 days interval to study the effect of culture passages on the explants response for shoot induction and multiple shoot formation. All the treatments were repeated at least three times with 10 replicates and data were subjected to statistical analysis.
RESULTS AND DISCUSSION
In the present study both apical and explants were used. But the nodal explants were found to be more effective for shoot induction and multiple shoot formation when culture on MS medium supplemented with various phytohormones as compared to other explants. The nodal explants of V.negundo L. were cultured on MS medium supplemented with various concentrations of BAP or KN individually or in combination with NAA or IAA resulted in induction of healthy shoots. When the explants were cultured on MS medium supplemented with cytokinins alone lesser number of shoots were induced in comparison to the MS medium supplemented with combination treatment of auxin and cytokinin.
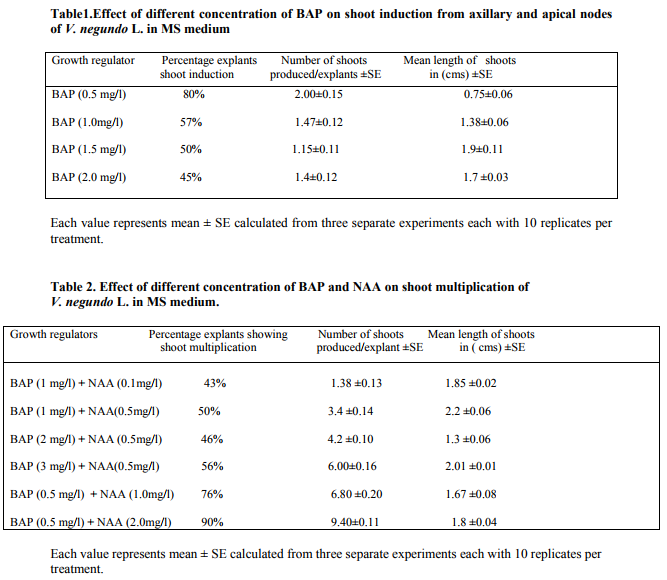
The explants were cultured on MS medium supplemented with sucrose (30gm/L.) along with optimal concentrations of BAP (0.5mg/L.) and NAA (2mg/L.) which was found to be the most effective in the induction of shoots compared to other concentrations. In-vitro raised shoots (20-30days old) were sub cultured on MS medium supplemented with BAP (0.5mg/L.) and NAA (2mg/L.). The highest response of nodal explants (90%) with a maximum average number of shoots (3.40±0.11) per explant was observed. There have been several reports of micro propagation with nodal segment and shoot tips of tropical medicinal plants in the juvenile phase of development (Kukreja et al., 1988). Here the protocol is described for rapid and large scale propagation of the woody aromatic and medicinal shrub V.negundo by in-vitro culture of nodal segments from mature healthy plants. Here different concentrations of cytokinins were used as supplements to the MS medium. Among the cytokinins tested BAP was found to be most effective than other cytokinins for the induction of shoots. The bud breaking and shoot induction in cultures of nodal explants indicate the function of cytokinins (Sahoo and Chand 1998). In the present investigation bud breaking and multiple shoot induction was increased in treatments of BAP up to 0.5mg/L. However there was decline in shoot induction beyond this dosage. In each explant 4-6 axillary buds were formed within 15-20 days after inoculation. The number of shoot formation per explants was increased when the cultures were transferred to a fresh medium. The enhancing effect of MS medium supplemented with auxins and cytokinins in shoot multiplication was also studied on Gomphrena officinalis (Mereker et al., 1992) and on Rauvolfia serpentina. Similar observations were made by Sahoo and Chand (1998) in the shoot multiplication of V.negundo when sub cultured on MS medium supplemented with BA (4.40um/L.) and GA3 (1.15um/L) up to two subcultures and then there was a gradual decline. Similar results were found on shoot induction and multiple shoot formation from nodal explants of V.negundo in the combination treatment of BA (16.80um/L.) and IBA (2.25um/L.) supplemented with 100mg/L. silver nitrate. Noman et al., (2010) observed the high frequency bud initiation and shoot proliferation from callus by using BAP (0.3mg/L) and IAA (0.3mg/L). In the present study MS medium along with NAA and BAP has been used which has also been reported the best shoot proliferating combination in Heracleum candicans (Wakhlu and Sharma, 1999) Centella asiatica (Shashikala et al., 2005) and Cardiospermum halicacabum (Jawahar et al.,2008). In contrast Fraternale et al., (2002) reported that high concentration of auxin with cytokinin was stable for shoot multiplication in Bupleurum fruiticosum.


CONCLUSION
A simple and efficient method has been developed for shoot induction and multiple shoot formation and thus increasing the production of V. negundo. This is suitable for conservation of germplasm of this multipurpose medicinal plant species. Despite its economic importance the production of V. negundo is threatened by population growth, desertification, industrial development and attack by numerous parasites. The classical conservation techniques such as crossing over, Sexual and somatic hybridization and breeding give a genetically blind mixture. Propagation through vegetative cuttings is very slow and a large number of cuttings do not survive during transportation. It can also be propagated through seeds or root suckers. Poor viability of the seeds and the production of root sucker is strictly age dependent. The biotechnological approach such as plant tissue culture initiated from medicinal plants is a variable method for the large scale production of economically and medicinally important plants. The present study was undertaken to standardize a protocol for high frequency induction of multiple shoots from different explants and to regenerate plants of V.negundo to meet its demand in medicine and agriculture.
References:
1. Abu Shadat Mohammod Noman, Mohammod Sayeedul Islam, Nurul Alam Siddique and Khaled Hossain, 2008. High frequency induction of multiple shoots from nodal explants of Vitex negundo using Silver nitrate.Int.J.Agri.Biol. 10: 633-7.
2. Achari, B., Chowdhury, U.S., Dutta, P. K and Pakrashi, S. C, 1984. Two isomeric flavonones from Vitex negundo Linn. Phytochemistry, 23: 703.
3. Adnaik, R.S., Pai, P.T., Mule, S.N., Naikwade, S.N. and Magdum, C.S. 2008. Laxative Activity of leaves of Vitex negundo. Asian J. Exp. Sci.22: 159-160.
4. Alam, M.I and A.Gomes, 2003.Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts.j. Ethnopharmacol. 86; 75-80.
5. Anonymous. Directory of Indian medicinal plants (1992). Lucknow, CIMAP .49.
6. Asaka, Y. and Rana, A.C., 1973. Arch. Pharm. Res.14 (1):96-98.
7. Avadhoot, Y. and Rana, A.C. Abu (1991) worked on hepatoprotective effect of Vitex negundo against carbon tetra chloride induced liver damage.
8. Azahar-ul-Haq. Malik. A., Anis, I., Khan, S.B., et al. 2004. Enzyme inhibiting lignans from Vitex negundo. Chem. Pharm. Bull. 52: 1269-1272.
9. Babu, T.D., Kuttan, G., Paddikkala, J. 1995. Cytotoxic and antitumour activity of certain taxa of umbeliferae with special refrence to Centella asiatica (L.) Urban journal of ethno pharmacology. 48: 53-57.
10. Baral, S.R. and Kurmi, P.P.2006. A compendium of Medicinal plants in Nepal.
11. FraternaleD, GiamperiL, RicciD, and RocchiMBL (2002).Micropropagation of B. fruticosum: The effect of triacontanol, plant cell tiss. Org. Cult.69: 135-140.
12. Kukreja AK., AK, Mathur. PS, Ahuja and RS, Thakur, (1988). Tissue culture and Biotechnology of Medicinal and Aromatic plants, pp: 7-11. CIMAP ,Lucknow,India
13. Shashikala CM, Shashidhara S and Rajeshkharan PE (2005). In-vitro regeneration of Centella asiatica L. Plant cell Biotech and Mol. Biol.6:53-56.
14. Wakhlu AK and Sharma RK (1999). Micropropagation of Heracleum candicans wall. A rare medicinal herb. Soc. In vitro Biol. 98: 1071-1074.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License