IJCRR - 4(12), June, 2012
Pages: 224-232
Date of Publication: 22-Jun-2012
Print Article
Download XML Download PDF
STRATEGIC ANALYSIS OF INDIAN NUTRACEUTICAL REGULATORY SCENARIO THROUGH MARKET RESEARCH
Author: Ajay Pise, Shilpa Pise, D. Sreedhar, Manthan Janodia, Virendra Ligade, Udupa N.
Category: Healthcare
Abstract:Objective of study: To analyse Indian Nutraceutical regulatory scenario. Research Methodology: A questionnaire was designed including open and closed ended questions. Data was collected through primary data collection method by visiting retail stores to know about the Nutraceutical product in selected area. Convenient, non-probability sampling process was adopted for the study. Sampling Unit: retail shops, medical shops, malls from selected localities of southern Karnataka. Sampling size of 70 was calculated by standard formula to achieve desired confidence level of 97% . Percentage analysis method was adopted for Data Analysis, Statistic calculator (version 3.0) by StatPac Company was used for data processing. This study has revealed that, child Nutraceutical Products are in high demand followed by Nutraceuticals for Pregnant / Lactating Mothers and Weight Gain Products therefore, it is very important to regulate the manufacturing, labeling, and advertisement of the Nutraceutical products targeted to Children, Pregnant and lactating mothers. Increasing trends in preventive therapy is a major driving force for nutraceutical market followed by increase in self medication. It is also important to regulate the advertisement of Nutraceuticals to restrict misleading claims in advertisements. In order to increase the sale of Nutraceutical product, it is advised that, manufacturers should reduce the price of product and make more efforts to spread awareness about the benefits of nutraceutical product. This study reveals that, Nutraceutical manufacturer have tremendous opportunities to explore Nutraceutical market.
Full Text:
INTRODUCTION
We understand that global Nutraceutical market is growing very rapidly. In the context of Indian scenario, Nutraceutical market is growing fast with the 20% CAGR5. Increasing awareness about the health, disease prevention, high cost of the medication, and handy disposable income are the factors which contributed in increasing demand of the Nutraceuticals. To explore this market, many Nutraceutical and Pharmaceutical companies have launched nutraceutical products in the healthcare market. Today, healthcare market in India is flooded with Nutraceutical products. Absence of proper regulatory framework and dilemma over the understanding and adopting the term ?Nutraceuticals? has became undue advantage for Nutraceutical manufacturers. Manufacturing, Labeling, Sale and Advertisement of such products needs proper regulatory framework to safeguard the interest of customers and control the Nutraceutical market. It has been observed that there is no universally acceptable definition for Nutraceuticals. Several scholars have proposed different definitions. Also, there is a dilemma on classification of nutraceuticals and understanding the difference between Functional Food, Dietary Supplement, and Medicated Food. To avoid this dilemma, we have attempted to propose definition and classification of Nutraceuticals. This study focuses on understanding and analysis of regulatory issues related to nutraceuticals in Indian Nutraceutical market.
RESEARCH METHODOLOGY
Data was collected through primary data collection method by visiting retail stores to know about the Nutraceutical product in selected area. Convenient, non-probability sampling process was adopted for the study. Sampling Unit: retail shops, medical shops, malls from selected localities of southern Karnataka. Sampling size of 70 was calculated by standard formula to achieve desired confidence level of 97% .
Sampling Size calculation:
Best estimate of the population size = 1500 Best estimate of the rate in the population (%) = 6% Maximum acceptable difference (%) = 6% Desired Confidence level of result (%) = 97% Required Sample size for desired confidence level=70 Data Analysis and Statistical Tool: Percentage analysis method was adopted for Data Analysis, Statistic calculator (version 3.0) by StatPac Company was used for data processing.
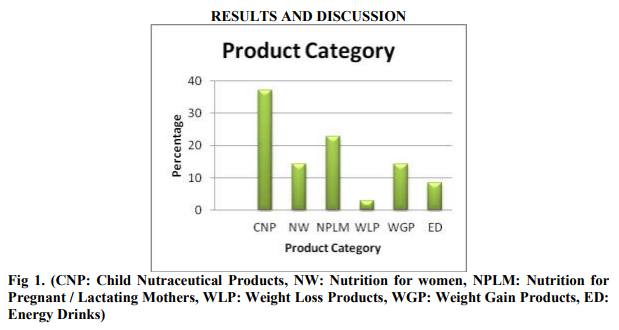
Data presented in above table suggests that Child Nutraceutical Products are in high demand followed by Nutraceuticals for Pregnant / Lactating Mothers and Weight Gain Products. Children and Pregnant mothers are considered as vulnerable group of population. Therefore it is very important to regulate the manufacturing, labeling, and advertisement of the Nutraceutical products targeted to Children, Pregnant and lactating mothers.
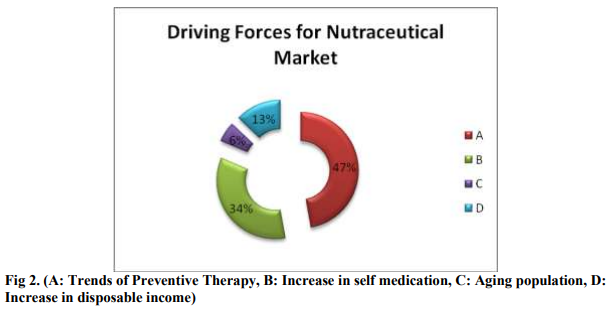
Above information suggests that increasing trends in preventive therapy is a major driving force for nutraceutical market followed by increase in self medication. It is observed that people are becoming more cautious about their health and tends to use nutraceutical products to prevent disease. Nutraceutical products are also popular among ?Baby Boomers‘. Increase in the disposal income is also one of the major driving forces for Nutraceutical market.
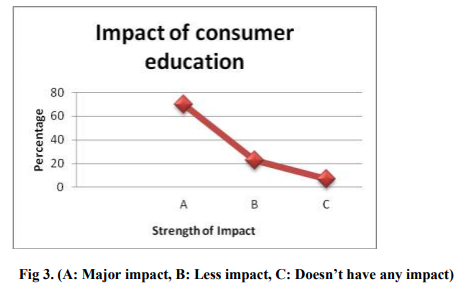
Data presented in above graph reflects that advertisement plays important role in consumer education about the product. Therefore, we can interpret that advertisement is an effective tool for product promotion. It is important to regulate the advertisement of Nutraceuticals to restrict misleading claims in advertisements
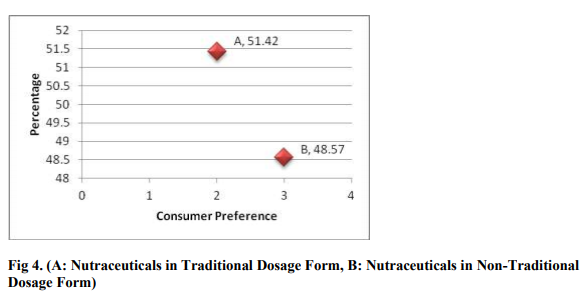
From the above data we can interpret that majority of consumers prefer to buy Nutraceutical products in traditional dosage form. Traditional dosage form includes capsule, tablet, solution, etc. Whereas nontraditional dosage form include mixture (liquid and solid), gels, etc.
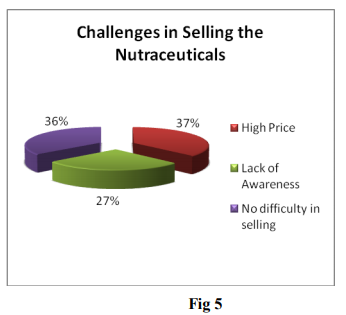
From the above graphical presentation of collected data we can conclude that High Price of the nutraceutical products is major challenge for its sale. Some group of respondents is of the opinion that lack of awareness about the benefits of nutraceutical products is also major challenge in selling Nutraceutical product. In order to increase the sale of Nutraceutical product, manufacturers should reduce the price of product and make more efforts to spread awareness about the benefits of nutraceutical product.
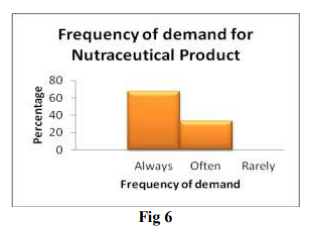
This question is posed to analyse frequency of demand for nutraceutical products. Above data suggests that nutraceutical products are always in demand. This shows market growth for nutraceutical products. Nutraceutical manufacturer have tremendous opportunities to explore Nutraceutical market.
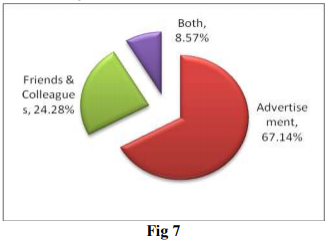
This question is posed to know about the factors influencing decision of customers. Data presented in above graph reveals that advertisement of the product is major factor to influence the decision of consumer. Advertisement includes product promotion through print, oral, electronic media. Word of mouth publicity through friends, colleagues is another influencing factor. Therefore, it is important for the Nutraceuticals manufacturer to give more emphasis on advertisement while promoting the product. It is also important to regulate advertisement of Nutraceutical products.
CONCLUSION
After analyzing information and data from the above study, we can derive following conclusionGlobal Nutraceutical market is growing very rapidly. In context of Indian scenario, Nutraceutical market is growing fast with the 20% CAGR. Increasing awareness about the health, disease prevention, high cost of the medication, and handy disposable income are the factors which contributed in increasing demand of the Nutraceuticals. Nutraceutical market is considered as one of the fastest growing market. Therefore it is important to regulate this market to safeguard the interest of consumers.
Nutraceuticals, around the world, falls under a grey area. Most of the food and Pharmaceutical companies are using term ?Nutraceuticals‘ as marketing gimmick. Though the Nutraceutical market is growing with fast rate and demand is increasing rapidly, no specific regulations are framed for Nutraceuticals in India. Absence of proper regulatory framework for manufacturing, labeling, advertising and sales of the nutraceuticals has became undue advantage for Food and Pharmaceutical companies to launch Nutraceutical products in healthcare market. It has been observed that there is no universally acceptable definition for Nutraceuticals. Several scholars have proposed different definitions, but essence of all definition remains same ?Food as Medicine‘. There is a dilemma on classification of nutraceuticals and understanding the difference between Functional Food, Dietary Supplement, and Medicated Food. To avoid this dilemma, we have proposed a definition of Nutraceuticals which can be adopted widely. In order to regulate the manufacturing of Nutraceutical products, Nutraceuticals can be classified in two broad categories i.e. Regular Nutraceuticals and Novel Nutraceuticals. Regulatory requirements for manufacturing of Regular Nutraceuticals and Novel Nutraceuticals should be different. It should not be compulsory to get manufacturing approval from FDA for regular nutraceutical product. But the product should be registered with FDA (at regional / district level) before launching it in the nutraceutical market. Child Nutraceutical Products are in high demand followed by Nutraceuticals for Pregnant / Lactating Mothers and Weight Gain Products therefore, it is very important to regulate the manufacturing, labeling, and advertisement of the Nutraceutical products targeted to Children, Pregnant and lactating mothers. I#ncreasing trends in preventive therapy is a major driving force for nutraceutical market followed by increase in self medication. It is also important to regulate the advertisement of Nutraceuticals to restrict misleading claims in advertisements. In order to increase the sale of Nutraceutical product, it is advised that, manufacturers should reduce the price of product and make more efforts to spread awareness about the benefits of nutraceutical product. This study reveals that, Nutraceutical manufacturer have tremendous opportunities to explore Nutraceutical market.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Ekta K. Kalra, ?Nutraceutical - Definition and Introduction? cited from http://www.aapspharmsci.org/view.asp?art =ps050325.
2. P A Francis, ?A Regulation for Nutraceuticals? cited from http://www.pharmabiz.com/article/detnews. asp?articleid=12303§ionid=47.
3. ?Industry Insight: Nutraceuticals? a market research report published by Cygnus Business Consulting and Research, Hyderabad. Published on February 2008
4. R. K. Rishi, ?Nutraceuticals: Borderline Between Food and Drugs? published in ?The Pharma Review‘ Vol 4 No. 20 (Feb. 06)
5. ?Market Status of Nutraceuticals? Cited From http://www.bccresearch.com/food/GA085R .html.Accessed on 12th Apr 2006
6. V. D. Deshmukh, ?Nutraceuticals: Dietary Supplements a Legal Dilemma? IDMA 30th June 2005 no. 24, pg 38-39
7. Raja Prasanna, ?Nutraceuticals to Gain Ground Globally? Published in ?The Pharma Review‘ Vol 4 No. 20 (Feb. 06)
8. ?Nutraceuticals Market Review‘ cited at http://www.teknoscienze.com/agro/pdf/nov _dec03/bioceuticals.pdf. Accessed on 12th Apr 2006
9. ?Regulatory status? cited from http://www.globalregulatory.com/labclass/ nutraceutical_consultants.cfm. Accessed on 25 Apr 2006
10. ?Introduction of Nutraceuticals? cited at http://www.anajana.org/nut_info_details.cfm?NutInfoID=4 . Accessed on 25th Apr 2006
11. Presentation for FDA public hearing, June 8, 1999- Washington, DC by Gary L. Huber published in American Nutraceuticals Association newsletter.
12. ?Nutraceuticals‘ cited at http://chemistry.about.com/od/chemistryglo ssary/a/nutraceuticaldf.htm
13. ?Definition of Drug‘ cited at http://en.wikipedia.org/wiki/Drug
14. ?Definition of Drug‘ cited at http://www.answers.com/topic/drug
15. ?Industry Insights-Nutraceuticals? report published by Cygnus Business Consulting and Research, Hyderabad
16. ?Food Regulations? Published by FDA, available at http://www.fda.gov/Food/GuidanceCompli anceRegulatoryInformation/GuidanceDocu ments/FoodLabelingNutrition/FoodLabelin gGuide/ucm064904.htm#specific
17. P A Francis, ?Regulating Nutraceuticals? Published on October 28, 2009 available at http://www.pharmabiz.com/article/detnews. asp?articleid=52317§ionid=47
18. ?About Codex? available at http://www.fao.org/docrep/w9114e/W9114 e04.htm#
19. ?Food Safety Standards Act 2006? Published by Ministry of Law and Justice, Government of India. Published on 24 Aug 2006
20. Om P. Gulati, Peter Berry Ottaway, ?Legislation relating to nutraceuticals in the European Union with a particular focus on botanical-sourced products? Published in Journal of Toxicology, 221 (2006) Page No. 75–87
21. Nisha Kaushik, Deepak Kaushik ?Functional Food/Nutraceuticals Regulation In India? Published on http://www.pharmainfo.net/reviews/functio nal-foodnutraceuticals-regulation-india
22. Gil Hardy ?Nutraceutical and Functional Food: Introduction and Meaning?, Nutrition, 2000, volume 16, 688-689.
23. ?Regulation of functional food in Indian Subcontinent?, available at http://www.efenbeonline.com/view_story. asp? type=storyandid=880
24. ?New Food Words: Functional Foods and Nutraceuticals, Phytochemicals? available at http://www.extension.iastate.edu/ publications/PMI846.pdf
25. ?India together: Legislative Brief?, available at http://www.indiatogether.org/2006/feb/law s- foodsafe/htmal/hilite).
26. FICCI study on Implementation of Food Safety and Standard Act 2006: An Industry Perspective at http://www.indiaenvironmentportal.org.in/ Files/food_safety_study.pdf)
27. Ashriti K K, ?Supplementary Growth? Published in ?Express Pharma‘, February 16-29,2008.
28. Akshay G Mehta, ?Untapped Wealth of Nutraceutical Exports?, Published in ?The Hindu Business Line‘, August 15, 2008.
29. ?Defining Functional Foods? Published in http://www.asiafoodjournal.com/article5565-definingfiunctionalfood claimsAsia.html.
30. Bijan Mukherjee, ?India holds potential to be a nutraceutical powerhouse? Published in http://www.pharmabiz.com/article/detnews. asp?articleid=52859§ionid=50
31. Clare Hasler, ?Regulation of Functional Foods and Nutraceuticals: A Global Perspective? (institute of Food Technologists Series) Vol 3, Published by Goteborgs University Published in 2008
32. ?Procedural Manual for the Codex India?, First Edition, Published by Ministry of Health and Family Welfare, Government of India
33. The Prevention of Food Adulteration Act and Rules 2004, Published by Ministry of Law and Justice, Government of India. Published on 01 Oct 2004
34. Rong Tsao, M. Humayoun Akhtar, ?Nutraceuticals and functional foods: II. Current International Regulatory Status? Published in Journal of Food, Agriculture and Environment Vol.3 (1): 18 - 20. 2005
35. Barry L. Smith, Michelle Marcotte, Gordon Harrison ?A Comparative Analysis of the Regulatory Framework Affecting Functional Food Development and Commercialization in Canada, Japan, the European Union and the United States of America? Published on March 31, 1996
36. ?Definitions? Published by Shambrock Consulting Group Inc. and Kelwin Management Consulting, available at http://www4.agr.gc.ca/AAFC-AAC/display afficher.do?id=1171305207040andlang=eng #s3
37. Esther Bull, Lisa Rapport, Brian Lockwood, ?Nutraceuticals?. Published in Pharmaceutical Journal 2000; 265(7104):57-58
38. C.K Kokate, A.P Purohit, S.B Gokhale, ?Nutraceuticals and Cosmeceuticals?. A textbook of Pharmacognosy. 36th edition. Published by Nirali Prakashan Pune; 2006. p. 542-543.
39. ?Identification and Analysis of Raw Materials for Nutraceutical Industry? Available at http://www.osun.org/raw+ animal+material-ppt.html
40. ?Nuttraceuticals and Functional Food Regulations in United States and Around the world? Edited by Debasis Bagchi
41. Devaraj S , Jialal I , Vega-Lopez S. ?Plant sterol-fortified orange juice effectively lowers cholesterol levels in mildly hypercholesterolemic healthy individuals? Published in Arterioscler Thromb Vasc Biol 24, e25 – e28
42. Devaraj S, Autret BC, Jialal I, ?Reducedcalorie orange juice beverage with plant sterols lowers C-reactive protein concentrations and improves the lipid profile in human volunteers? American Journal of Clinical Nutrition 84, 756 – 761
43. Ridinger MHT ?Nutraceuticals: miracle or meme?? Published in ?Clinical Pharmacology Therapeutics? 82, 352 – 356
44. Nielsen Global Survey, ?Word-of-mouth the most powerful selling tool? available at http://www.nielsen.com/media/2007/pr_07 1001.html
45. Leibenstein H., Bandwagon, Snob, and Veblen ?Effects in the Theory of Consumers‘ Demand? Published in Quartly Journal of Economics 64, 183 – 207.
46. Smith RN , Mann NJ , Braue A , Makelainen H , Varigos GA. ?The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigatormasked, controlled trial? Published in Journal of American Academic Dermatology 57, 247 – 256
47. Poulin Y, Bissonnette R, Juneau C, Cantin K, Drouin R, Poubelle P E. ?XP- 828L (Dermylex ™ ) in the treatment of mild-tomoderate psoriasis: a randomized, double blind placebo-controlled study?. Published in Journal of Cutanious Medical Surgery 10, 241 – 248
48. Osmo Hanninen, Chandan K. Sen, ?Nutritional Supplements and Functional Foods: Functional Significance and Global Regulations? Published in ?Nuttraceuticals and Functional Food Regulations in United States and Around the World? Edited by Debasis Bagchi, Page No. 11-27
49. Morrow JD , Edeki TI , El Mouelhi M, ?American Society for Clinical Pharmacology and Therapeutics Position Statement on Dietary Supplement Safety and Regulation? Published in Clinical Pharmacology Therapeutics 77, 113 – 122 50. Yen P.K., ?Food and Supplement Safety? Published in Geriatric Nurs 26, 279 – 280
51. Ziker D., ?What lies beneath: an examination of the underpinnings of dietary supplement safety regulation? Published in American Journal of Law Medicine 31, 269 – 284.
52. Melby CL, Toohey ML, Cebrick J. ?Blood pressure and blood lipids among vegetarian, semivegetarian, and nonvegetarian African Americans? Published in American Journal of Clinical Nutrition 59, 103 – 109.
53. Richardon DP, Group IS. ?Nutrition, health ageing and public policy Brussels? Presented in the proceedings of International Alliance of Dietary Food Supplement Associations (IADSA), pp. 1–71.
54. Agren JJ , Hanninen O , Julkunen A. ?Fish diet, fish oil and docosahexaenoic acid rich oil lower fasting and postprandial plasma lipid levels? Published in European Journal of Clinical Nutrition 50, 765 –771.
55. Smith BK, Sun GY, Donahue OM, Thomas TR. ?Exercise plus n-3 fatty acids: additive effect on postprandial lipemia? Published in Metabolism 53, 1365 – 1371.
56. Ruzickova J, Rossmeisl M, Prazak T. ?Omega-3 PUFA of marine origin limit dietinduced obesity in mice by reducing cellularity of adipose tissue?. Published in ?Lipids‘ 39, 1177 – 1185.
57. Ghafoorunissa, Ibrahim A, Rajkumar L, Acharya V. ?Dietary (n-3) long chain polyunsaturated fatty acids prevent sucroseinduced insulin resistance in rats? Published in Journal of Nutrition 135, 2634 – 2638.
58. Whelan J, Li B, Birdwell C. ?Dietary arachidonic acid increases eicosanoid production in the presence of equal amounts of dietary eicosapentaenoic acid? Published in Adv Exp Med Biol 400B, 897 – 904.
59. Schuman BE, Squires EJ, Leeson S. ?Effect of dietary flaxseed, flax oil and n-3 fatty acid supplement on hepatic and plasma characteristics relevant to fatty liver haemorrhagic syndrome in laying hens? Published in ?British Poultry Sciences‘ 41, 465 – 472
60. Drain PK, Kupka R, Mugusi F, Fawzi WW. ?Micronutrients in HIV-positive persons receiving highly active antiretroviral therapy? Published in American Journal of Clinical Nutrition 85, 333 – 345
61. Neumann CG. ?Symposium: food-based approaches to combating micronutrient deficiencies in children of developing countries. Background? Published in ?Journal of Nutrition‘ 137, 1091 – 1092
62. Naresh K. Malhotra, Textbook on Marketing Research an Applied Orientation, 3rd Edition, Person Education, India.
63. David A Aaker, V Kumar, George S. Day, Textbook on Marketing Research, 7th Edition, John Willy and Sons Inc.
64. Manisha Pandey, Rohit K Verma, Shubhini A Saraf, ?Nutraceuticals: New Era of Medicine and Health?, Published in ?Asian Journal of Pharmaceutical and Clinical Research‘ JanMarch 2010, Vol.3 Issue 1
65. Dr K Bhaskaran, ?Nutraceuticals? Published in ?Health Administrator‘ Vol:XX Number1and2, Pg 76-77, available at www.medind.nic.in/haa/t07/i1 /haat07i1p76.pdf
66. FMHG Market in India- A Close View, Published in Express Pharma 16-31 March 209, available at www.expresspharmaonline.com
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License