IJCRR - 4(12), June, 2012
Pages: 118-124
Date of Publication: 22-Jun-2012
Print Article
Download XML Download PDF
EFFECT OF TANK COLOURATION ON THE SURVIVAL, GROWTH AND DEVELOPMENT RATE OF BLUE SWIMMING CRAB, PORTUNUS PELAGICUS (LINNAEUS, 1758) LARVAE
Author: Azra M. N, Wendy W, Talpur A.D, Abol-Munafi A. B, Ikhwanuddin M
Category: General Sciences
Abstract:The purpose of this study was to investigate the effect of background tank colouration on survival, growth, and development rate of Portunus pelagicus larvae. The results demonstrated that highest survival was achieved in black background colour tanks was statistically significantly different (p< 0.05). However, specific growth rate (SGR) was observed higher in red colour tanks over the other treated groups was statistically significantly different (p< 0.05). The results also demonstrated that the darker background colour of black and red took 9 days to reach Zoea 4 stage compared to the other treatments
background colour tanks, which took 10 days. Meanwhile, the SGR for the treatments of white, orange, and yellow background colour were not determined because none of the Zoea 4 stage from these treatments reached to Megalopa stage. The black background colour is favourable for P. pelagicus larvae
rearing to ensure the highest survival, growth and development rate.
Keywords: Background, Larval rearing, Megalopa, Survival, Portunus pelagicus, Zoea
Full Text:
INTRODUCTION
Blue swimming crab, Portunus pelagicus has been collected for years from intertidal zone, estuaries, shallow inshore and jetties in coastal areas in various part of the world. Therefore, the demand for the crab have been exceeded the capacity of the crab fishery [1]. Consequently, to facilitate continued growth of the crab industry especially in Malaysia, the development of commercial seed production technology, which would improve the survival, is required. The seed production development of P. pelagicus in Malaysia is in the trial stages. Until date, P. pelagicus for local consumption or for culture is caught from the wild [2]. Hence, further research on seed production technology of P. pelagicus would be considered as the way to increase P. pelagicus seeds that would solve the problems associated with fishery catches in order to maintain environment ecology sustainable. Many countries like Japan, Philippines, India, Indonesia, Thailand, Bangladesh, Vietnam, Australia and USA are actively involved in crab culture and research [3]. Since, the P. pelagicus aquaculture is very new in Malaysia, with no appropriate techniques established for the commercial production of juvenile crabs for P. pelagicus [4, 5]. Castine et al. [6] reported that current seed production for Portunid decapods is inconsistent. Due to the lack of necessary biological information required for the development of appropriate grow-out techniques, the industry is still in the developing phase. Extensive information on the culture techniques is still required to develop sustainable seed production of P. pelagicus crab. There are few studies on the effects of tank coloration on crustacean larvae such as in prawn [7, 8] has been conducted. Nevertheless, there is only one specific work on hatchery-rearing techniques for juvenile‘s production of Portunid crab by using four different tank colours by [8]. The prime objective of this study was to investigate the effect of tank colouration on survival, growth, and development rate in early stages of the P. pelagicus larvae.
MATERIALS AND METHOD
Seawater for broodstock and larviculture
Seawater for crab culture was treated according to Talpur et al. [9]. UV treated seawater was filtered through a 10 µm net and then disinfected with active chlorine for 24 h. This procedure, which eliminated almost all naturally occurring bacteria, treated water was supplemented EDTA (after 24 h of chlorine treatment) to settle down the heavy metals was followed by neutralization with sodium thiosulphate (same concentration of chlorine) at the beginning of the experiment. The culture water exchange began from the day second, using disinfected seawater.
Water parameter
The water parameters for broodstock and larviculture were maintained as constant including salinity 30 ppt, pH > 8.0, temperature at 30 ºC using 110-V heater and dissolved oxygen (DO) > 5 mg L-1 during the conducting trials. Water parameters were measured daily on site with YSI 556 Multi-probe meter (USA). Broodstock Healthy berried females were collected from Strait of Tebrau, Johor, West Malaysia, (1o 22‘ N and 103o 38‘ E) and were transported to marine hatchery of Institute of Tropical Aquaculture, Universiti Malaysia Terengganu, Malaysia. Berried female was disinfected according to Talpur et al. [9] and one berried crab per (300 L) tank was placed in the hatching tank with 3 cm substrate of thick sand tray with adequate aeration and 100% water exchanged daily. During incubation period, the berried females were not fed. The berried females were monitored daily for hatching. After hatching, the larvae were transferred to another tank filled with disinfected seawater. Energetically moving larvae at surface were collected and used for stocking in rearing tanks. Stocking and feeding The larvae culture tanks used were 200 L capacity filled with 150 L disinfected seawater and the stocking density at 100 larvae L-1 , about 15,000 larvae per tank. Feeding scheme used for rearing P. pelagicus larvae from Zoea1 until Megalopa stages as described method by Ikhwanuddin et al. [2]. Larvae were fed with rotifers (Brachionus sp.) at the rate of 30-35 individuals mL-1 and Nannochloropsis sp. (5x106 cells mL-1 ) from Zoea 1 to Zoea 4 stage and. Artemia sp. nauplii were provided from Zoea 3 to Zoea 4 stages. The rotifers were cultured with microalgae Nannochloropsis sp., while Artemia sp. nauplii were hatched daily and harvested washed and fed directly to larvae. Larvae were fed once daily in the morning at about 10.00 A.M.
Identification of larval stages
The different crab larval stages from Zoea 1 to Megalopa stage were observed under profile projector based on morphological characters.
Experimental design
200 L plastic tanks were used for larval rearing and all tanks were sprayed with five different background colours including black, white, red, orange and yellow. All trails were conducted in three replicates. Control tanks were without any background colour. Total 15 larvae rearing batches were conducted in this study. Moderate aeration was provided in the larval rearing tanks. Throughout the study, 12 h light and 12 h dark photoperiod was maintained. Dead larvae are
removed daily to prevent contamination. Larvae numbers were estimated daily through volumetric method from the rearing tanks. Fifty percent of water was changed daily in larval rearing tanks. To remove left over feed, detritus, and dead larvae each day, the aeration stopped temporarily and settled particles were removed from the tank bottom by siphoning. The experiment terminated when all larvae had either died or metamorphosed to the Megalopa stage.
Specific growth rate (SGR)
Randomly sampled 10 larvae from each culture tank were collected for every Zoea stage for specific growth rate (SGR) study. Sampled crab larvae were put into disposal scintillation vials (Bjorn bottle) preserved in 10 % of formalin, and weighed under the microbalance. The mean BW for each treatment for different larval stages was calculated to determine the specific growth rate (SGR) using following formula:

Statistical analysis of data
Effects of treatments on water quality, larvae survival and growth were evaluated by analysis of variance (ANOVA) using SPSS for Windows, version 16.0 at significant differences p<0.05, comparisons between treatments were compared followed by Post Hoc using Tukey‘s test.
RESULTS
Survival rate
The mean survival rate of crab larvae from Z1 to Z2 stages were 23.5%, 25.5%, 26.8%, 21.5% % and 13.5% % for larvae rearing with black, white, red, orange and yellow background colour tanks respectively. The highest mean survival rate 11.50 % of crab larvae from Z2 to Z3 stages were observed in black background colour tanks over the white, red, orange and yellow background colours with mean survival rate of 9.5%, 7.0%, 3.8% and 2.5% respectively Table 1. Treatment with black background colour did produce the highest mean survival 8.3% of Z3 to Z4 crab larvae compared to white, red, orange, and yellow background colour treatments Table 3. The result showed that only the black and red background colour treated groups afforded Z4 to Megalopa stages survival at 4.5% and 1% respectively Table 3. Statistics analysis of variance showed that survival was significant difference (p<0.05) between the treated groups.
TABLE-1
Growth rate
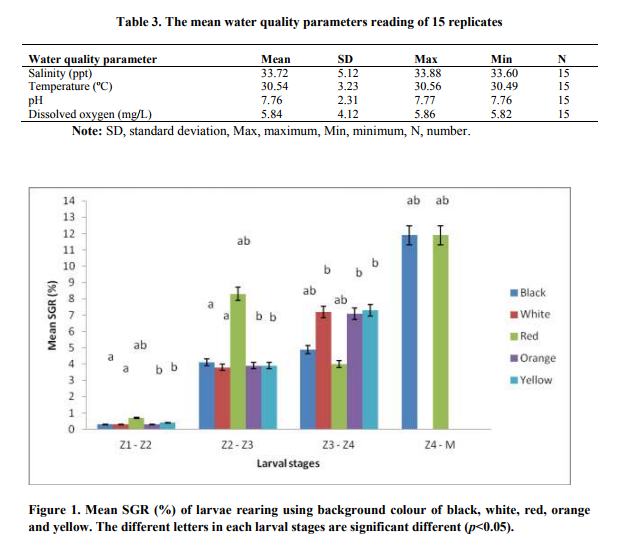
The highest SGR 8.3% was observed in Z2 and Z3 followed by 0.7% in Z1 and Z2 stages in red colour background tanks respectively Figure 1. However, the result demonstrated that the black and red background colour treatments did produce highest SGR 11.9% in Z4 and Megalopa stage respectively. For the other three treatments including white, orange, and yellow background colours were not determined because none of the Z4 stage from these treatments reached to Megalopa stage Figure 1.
FIGURE-1
Development rate
The larvae reared in black, white, red, orange and yellow background colour tanks reached up to Z2 stage on day 4 with LSI 1.8 ± 0.6, 1.5 ± 0.9, 1.7 ± 0.5, 1.5 ± 0.4 and 1.5 ± 0.8 respectively. Larvae reached to Z3 stage on day 7 with LSI 2.8 ± 0.8, 2.5 ± 0.5, 2.7 ± 0.8, 2.5 ± 0.8 and 2.5 ± 0.3 respectively Table 2. The larvae in black and red colour background took 9 days to reach Z4 stage with LSI 3.7 ± 0.8 and 3.6 ± 0.9 respectively. Thus, larvae in white, orange and yellow background tanks took 10 days to reach Z4 after hatching with LSI 3.8 ± 0.5, 3.7 ± 0.5 and 3.7 ± 0.5 respectively Table 2.
TABLE-2
Water quality parameter
Important water parameters such as salinity, temperature, pH, and dissolved oxygen were recorded daily on the site. The water parameters in treated groups were remained under acceptable ranges are shown in Table 3.
DISCUSSION
Survival in black and red culture tanks were statistically higher than the white, orange and yellow culture tanks; this is in agreement with the findings of Yasharian et al. [10] on freshwater prawn, Macrobrachium rosenbergii when reared in red and green tanks showed best larval survivals compared to white and blue tanks. The survival rate of crab larvae from Z1 until Megalopa stages in black background colour was significantly higher compared to the other colour treatments; perhaps it is the favourable colour for survival and growth of crab larvae. Study by Tume et al. [7] with black tiger prawn, Penaeus monodon, showed that visual appearance was affected by the background colour of the tanks particularly black tanks in which the prawns were growing better. The crab larvae reared in the black and red coloured background during the present study did show shorter development periods of 12 days to reach Megalopa stage compared to the other coloured background including white, orange and yellow, which did not reach Megalopa stage in 12 days. In another study by Rabbani and Zeng [8] also showed that S. serrata larvae culture tank background colours had significant effect on the larvae survival until 1st day juvenile crab C1 stage, where crab larvae reared in black tank did produce significantly higher survival rate than those reared in white tank . The later study had also showed to affect the larvae development where the crab larvae reared in darker background generally had shorter development period and more synchronised moulting. The results of the present study are similar to the study by Rabbani and Zeng [8] where darker coloured background produced the better results compared to the lighter coloured background. It can be explained that the darker coloured background may possibly facilitated more efficient feeding, reduced settlement of larvae at the bottom of the vessels and as well as minimizing stress for the crab larvae resulted better survival and growth.
CONCLUSION
The results concluded that the culture tank‘s background colour had significant effect on the survival, growth, and development rate to the next crab larval stage. The darker coloured background particularly black and red did show the better survival, growth and development rate over the lighter coloured background including white, orange and yellow. The results also demonstrated that white background colour produced the poorest result compared to the other four colour treatments. Based on results of the present study, it is recommended that the black background colour should be used for P. pelagicus larvae rearing to ensure the highest survival, growth and development rate.
ACKNOWLEDGEMENT
This research was supported by a grant from the Ministry of Science, Technology and Innovation (MOSTI) (Science Fund), government of Malaysia under grant Vote No. 52042. Researchers also wish to extend their thanks to Prof. Dr. Anuar Hassan the Director of AKUATROP, Universiti Malaysia Terengganu for providing facilities for the study.
References:
1. Ikhwanuddin, M., Shabdin, M.L. and AbolMunafi, A.B. 2009. Catch Information of Blue Swimming Crab (Portunus pelagicus) From Sarawak Coastal Water of South China Sea. Journal of Sustainability Science and Management. 4 (1): 93-103.
2. Ikhwanuddin, M., Nor Adila, T., Azra, M.N., Hii, Y.S., Talpur, A.D. and AbolMunafi, A.B. 2011. Determination of Live Prey Ingestion Capability of Blue Swimming Crab, Portunus pelagicus (Linnaeus, 1758) Larvae. World Journal of Fish and Marine Sciences. 3 (6): 570-575.
3. Soundarapandian, P., Thamizhazhagan E. and Samuel N.J. 2007. Seed production of commercially important blue swimming crab Portunus pelagicus (Linnaeus). Journal of Fisheries and Aquatics Science 2(4): 302-309.
4. Ikhwanuddin, M., Azra, M.N., Yeong, Y.S., Abol- Munafi, A.B. and Shabdin, M.L. 2012. Live Foods for Juveniles‘ Production of Blue Swimming Crab, Portunus pelagicus (Linnaeus, 1766). Journal of Fisheries and Aquatic Science. 7(4): 266-278.
5. Ikhwanuddin, M., Azra, M.N., Talpur, A.D., Abol- Munafi, A.B. and Shabdin, M.L. 2012. Optimal Water Temperature and Salinity for Production of Blue Swimming Crab, Portunus pelagicus 1st Day Juvenile Crab. Aquaculture, Aquarium, Conservation & Legislation. 5 (1): 4-8.
6. Castine, S., Southgate P.C. and Zeng, C. 2008. Evaluation of four dietary protein sources for use in microbound diets fed to megalopae of the blue swimmer crab, Portunus pelagicus. Aquaculture 281: 96- 99.
7. Tume, R.K., Sikes, A.L., Tabrett, S. and Smith, D.M. 2009. Effect of background colour on the distribution of astaxanthin in black tiger prawn (Penaeus monodon): Effective method for improvement of cooked colour. Aquaculture. 296: 129-135.
8. Rabbani, A.G and Zeng C. 2005. Effects of tank colour on larval survival and development of mud crab, Sylla serrata. Aquaculture Research. 36: 1112-1119.
9. Talpur, A.D., A.J. Memon, M.I. Khan, M.M. Ikhwanuddin, M. Danish Daniel and A.B. Abol-Munafi (2011). A Novel of Gut Pathogenic Bacteria of Blue Swimming Crab Portunus pelagicus (Linnaeus, 1758) and Pathogenicity of Vibrio harveyi a Transmission Agent in Larval Culture under Hatchery Conditions. Research Journal of Applied Sciences 6 (2): 116-127.
10. Yasharian, D., Coyle, S.D., Tidwell, J.H. and Stilwell W.E. 2005. The effect of tank colouration on survival, metamorphosis rate, growth and time to metamorphosis freshwater prawn (Macrobrachium rosenbergii) rearing. Aquaculture Research 36: 278-283.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License