IJCRR - 4(13), July, 2012
Pages: 119-126
Date of Publication: 18-Jul-2012
Print Article
Download XML Download PDF
EVALUATION OF IN-VIVO ANTI-RHEUMATIC ACTIVITY OF ANISOMELES MALABARICA R.BR.
Author: Ismail Shareef. M, Leelavathi. S, Thara Saraswathi. K. J, Sampath Kumara K. K
Category: General Sciences
Abstract:The present study was carried out to investigate the anti-rheumatic properties of methanolic extracts of the aerial parts of the plant Anisomeles malabarica R.Br., (Lamiaceae) using experimental animal models. The anti-rheumatic activity of the methanolic extracts was studied based on the effects on carrageenan-induced rat paw oedema. The preliminary phytochemicals were screened for the presence and absence of alkaloids, steroids, proteins, flavonoids, saponins, carbohydrates, tannins, fats and oils. The extracts in dose level 2000 mg/kg orally were used in anti-rheumatic studies. The methanolic extracts of leaves of Anisomeles malabarica R.Br., produced significant anti-rheumatic activity in a dosedependent manner (200 mg/Kg and 400 mg/Kg body weight) to that of standard drug indomethacin (10 mg/Kg). The extract exhibited inhibitory effect in carrageenan induced hind paw oedema in rats with all the doses used when compared to the control group. The data obtained indicate that the crude extracts of the aerial parts of the plant Anisomeles malabarica R.Br., possess potential anti-rheumatic activity by supporting the folkloric usage of the plant to treat various inflammatory conditions.
Keywords: Anisomeles malabarica R.Br., Anti-rheumatic, Carrageenan, Rat paw oedema
Full Text:
INTRODUCTION
Inflammation is a normal protective response to tissue injury that is caused by physical trauma, noxious chemicals or microbiological agents. Inflammation is the result of concerted participation of a large number of vasoactive, chemotactic and proliferative factors at different stages and there are many targets for anti inflammatory action1 . Rheumatoid arthritis is a systemic autoimmune disorder characterized by polyarticular symmetrical arthritis. Various inflammatory mediators produce joint inflammation with pain function loss, joint destruction and permanent deformity after certain time if remained untreated. This disease has a worldwide distribution but its pathogenesis is not clearly understood2 although there are few anti-rheumatic drugs showing effectiveness in the treatment of rheumatoid arthritis, the side effect and toxicity call for new and more effective natural drugs3 . There are many herbs which have been enlisted to have the potential in the symptomatic treatment of rheumatoid arthritis, hence the present study has been carried out to evaluate the anti-rheumatic potential. The tools of biotechnology provides vast potential for the development of new inventions, particularly in the field of pharmaceuticals which are environmentally safe and do not require heavy investments. In this context, the herbal medicines have been proved to have tremendous scope4 . Anisomeles malabarica R.Br. (Lamiaceae) is distributed in major parts of India and especially in South India as a traditional medicinal plant commonly known as Peymarutti (Tamil), Gouzaban (Hindi), Chodhara (Marathi), Karithumbi (Kannada) and Malabar catmint (English)5 . The herb is reported to possess antispasmodic, anti-periodic properties and used in rheumatoid arthritis6 . It is used for the traditional treatment of snakebite as antidote7 and plant leaves are used as carminative, astringent, stomachic, rheumatism and diaphoretic in Coimbatore district8 and also used as dentifrice to cure various problems9 . Preliminary phytochemical tests were carried out by Brindha et al., (1977)10 and used for the treatment of various infections.
MATERIALS AND METHODS
Chemicals
Extraction was carried out using methanol in soxhlet apparatus. Chemicals and reagents for the present analysis were purchased from Karnataka fine chemicals, Bangalore and E. Merck Ltd., Mumbai, India.
Plant material
Anisomeles malabarica was collected from Mysore, Nanjangud and surrounding areas and also from medicinal garden of Indian Institute of Horticultural Research, Hesaraghatta, Bangalore where they were growing profusely. The plants have been identified and authenticated by experts from National Ayurveda Dietetics Research Institute, Bangalore (Ref. No. SMPU/NADRI/BNG/2010-11/550).
Extraction of plant material
The plant materials were extracted with methanol using sohxlet extraction apparatus continuously for 16 hours11. For extraction, the dried plant material was used. Initially 400gms of material was packed in filter paper and loaded into the thimble of soxhlet apparatus. 2.5 liter of methanol was poured into the flask (distilling pot) and the whole apparatus was set. The soxhlet extraction was performed for 12- 16 hours until the collected solvent in siphon tube appears to be clear. Later the extracted solvent was evaporated under reduced pressure to get solid/ semi solid extract. The extract was weighed, physical characters were noted. The percentage yield was calculated to be 10.62.
Phytochemical screening
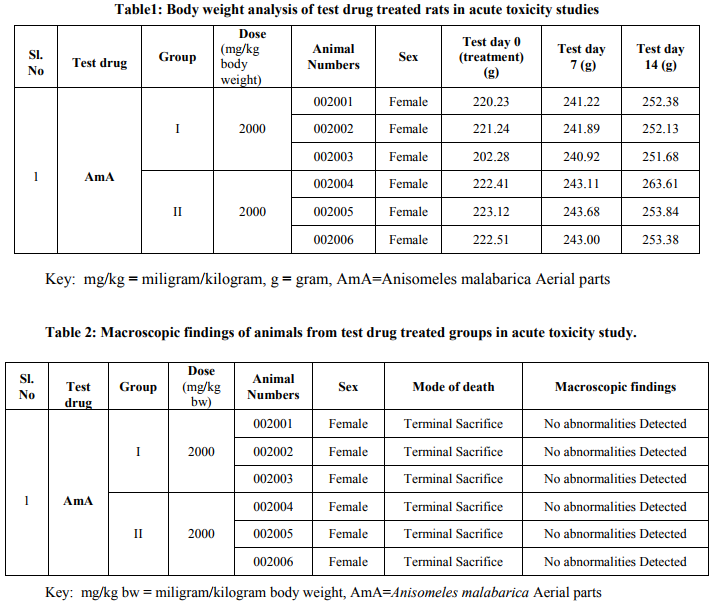
All the extracts were screened for the presence of various active plant metabolites like steroids, alkaloids, carbohydrates, flavanoids, glycosides and tannins according to standard phytochemical methods12. Briefly, Dragendorff reaction was used to confirm the presence of alkaloids, alkaline reagent test for tannins, frothing test for saponins, legal‘s test for glycosides, Xanthoproteic test for proteins and Shinoda test for flavanoids respectively. 1. Acute toxicity study in rats with test drugs13, 14 Two groups, each of three female rats, were treated with the extracts of the aerial parts of the plant namely Anisomeles malabarica (abbreviated as AmA/Test drug) by Oral administration at a dosage of 2000 mg/kg body weight. The test drug was formulated in vehicle (distilled water) at a concentration of 2000 mg/mL and administered at the dose volume of 10 mL/kg. The animals were observed daily during the acclimatization period and mortality/viability and clinical signs were recorded. All animals were observed for clinical signs during first 30 minutes and at approximately 1, 2, 3 and 4 hours after administration on test day 0 and once daily during test days 1-14. Mortality/viability was recorded twice daily during days 1-14 (at least once on day of sacrifice). Body weights were recorded on test day 0 (prior to administration), test days 7 and 14 (Table 1). All animals were necropsied and examined macroscopically (Table 2).
Treatment
The animals received a single dose of the test item by oral administration at 2000 mg/kg body weight after being fasted for approximately 18.0 hours but with free access to water. Food was provided again at approximately 3.0 hours after dosing. The administration volume was 10 mL/kg body weight. The animals were dosed using 18 G oral Stainless steel feeding tubes. Necroscopy All animals were sacrificed at the end of the observation period by carbon dioxide in euthanasia chamber and discarded after the gross/macroscopic pathological changes were observed and recorded. No organs or tissues were retained.
2. Anti-inflammatory studies15
This model is based on the principle of release of various inflammatory mediators by carrageenan. Oedema formation due to carrageenan in the rat paw is biphasic event. The initial phase is attributed to the release of histamine and serotonin. The second phase of oedema is due to the release of prostaglandins, protease and lysosome. Subcutaneous injection of carrageenan into the rat paw produces inflammation resulting from plasma extravasations, increased tissue water and plasma protein exudation along with neutrophil extravasations, all due to the metabolism of arachidonic acid. The pharmacological screening of the AmA was carried out using standard protocols. The crude extract was suspended in 1% carboxy methyl cellulose (CMC) for administration to albino rats. Albino rats of 150-200g were used for present investigation. They were kept in polypropylene cages in an air-conditioned area at 25 + 2oC in 10- 14 h light dark cycle. They were provided with Amrut brand balanced feed and tap water ad libitum.
3. Carrageenan induced rat paw oedema16
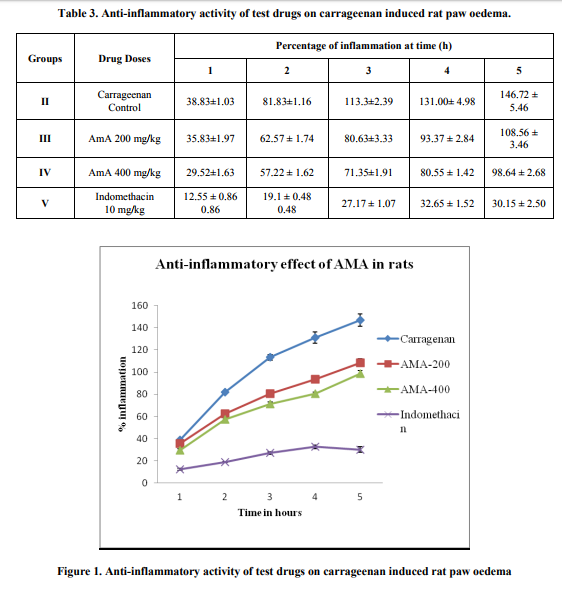
Thirty rats were divided into five groups (n=6) starved overnight with water ad libitum prior to the day of experiment. The group I kept as control group, group II kept as carrageenan control, groups III and IV received test drug at different doses and group V kept as standard drug control, respectively. Left paw was marked with ink at the level of lateral malleolus; basal paw volume was measured plethysmographically by volume displacement method using Plethysmometer (UGO Basile 7140) by immersing the paw till the level of lateral malleolus. In the experiment, animals from the control group were given vehicle control (CMC) and animals from standard drug were treated with Indomethacin as shown in Table 3. Other groups were treated with different doses of test drugs as shown in Table 3. After 30 min. of drug treatment the rats are challenged by a subcutaneous injection of 0.1ml of 1% solution of carrageenan into the sub-plantar side of the left hind paw. The paw volume is measured again at 1, 2, 3, 4 and 5 hours after challenge. The increase in paw volume is calculated as percentage compared with the basal volume. The difference of average values between treated animals and control group is calculated for each time interval and evaluated statistically. The percent Inhibition is calculated using the formula as follows. % oedema inhibition = [1- (Vt / Vc)] X 100 Vt and Vc are oedema volume in the drug treated and control groups respectively.
RESULTS
1. Acute toxicity study
All animals survived until the end of the experimental period. All the animals were dosed at 2000 mg/kg body weight did not show evident toxicity throughout the experimental period. The animals which were survived throughout the experiment increased their body weight by day 14 as compared to day 0. No abnormalities were detected in animals at necropsy. Based on the results, the median lethal doses (LD50) of AmA was greater than 2000mg/kg body weight and is classified as category 4.
2. Anti-inflammatory activity
In the control group, carrageenan induced significant inflammation over the normal untreated animals. AmA inhibited the inflammation significantly at the doses, 200 and 400 mg/kg at time 2, 3, 4 and 5 h. AmA dosed at 400mg/kg exhibited potent anti-inflammatory activity in dose dependant manner. Standard drug, indomethacin at 10 mg/kg inhibited the inflammation significantly at all time intervals (Figure 1). From these above findings it is evident that the aerial parts of the plant namely Anisomeles malabarica R.Br. possesses potent anti-rheumatic properties and it is further envisaged to carry out the purification of the bio-active compounds using column chromatography and elucidate the structure of the purified compounds using specialized spectral techniques like IR, MASS, C13 NMR and 1H NMR .
DISCUSSION
The carrageenan-induced paw oedema model in rats is known to be sensitive to cyclooxygenase inhibitors and has been used to evaluate the effect of non-steroidal antiinflammatory agents, which primarily inhibit the cyclooxygenase involved in prostaglandin synthesis17. Carrageenan-induced hind paw oedema is the standard experimental model of acute-inflammation. The time course of oedema development in carrageenan-induced paw oedema model in rats is generally represented by a biphasic curve18. The first phase of inflammation occurs within an hour of carrageenan injection and is partly attributed to trauma of injection and also to histamine, and serotonin components19. The second phase is associated with the production of bradykinin, protease, prostaglandin, and lysosome19. Prostaglandins (PGs) play a major role in the development of the second phase of inflammatory reaction which is measured at +3 h 20 . The dose 400 mg/kg of methanolic extract of Anisomeles malabarica R.Br. produced a significant inhibition of carrageenan induced paw oedema at +2, +3, +4, +5 and +6h. Therefore, it can be inferred that the inhibitory effect of methanolic extract of Anisomeles malabarica R.Br. on carrageenan induced inflammation could be due to inhibition of the enzyme cyclooxygenase and subsequent inhibition of prostaglandin synthesis. Significant inhibition of paw oedema in the early hours of study by Anisomeles malabarica R.Br. could be attributed to the inhibition of histamine21 and/or serotonin. The decrease in paw oedema inhibition at +6h may be attributed to the termination of test drug action.
CONCLUSION
It is evident from the above findings that the aerial parts of the plant namely Anisomeles malabarica R.Br. possess bio-active principles which are responsible in reducing the inflammation in carrageenan induced paw oedema in rats in dosed dependent manner. These findings also support the folkloric usage of the plant in treating rheumatoid arthritis and it becomes imperative to purify these bio-active principles using various purification methods like column chromatography and also repeat the same set of in-vivo studies in experimental models and also elucidate the structure of the purified compound and if found potent, chemically synthesize the compound.
On the basis of these findings, it may be inferred that methanolic extract of Anisomeles malabarica R.Br. has anti-inflammatory activities. These activities were related to the dose and these results corroborate the potential traditional use of the plant in folk medicine. At present, there are no reports on investigation to identify the active components present in methanolic extract of Anisomeles malabarica R.Br.. Further investigations are anticipated to identify the active components and lead to their further clinical use.
ACKNOWLEDGEMENT
The authors and the corresponding author acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. The authors are greatly thankful to Dr. Goli Divakar, Principal, Acharya and B. M. Reddy College of pharmacy for granting permission to use the animal facility to carry out the in-vivo studies of the test extracts and also Mr. Manjunatha. P. Mudagal, Assistant professor, department of Pharmacology, Acharya and B. M. Reddy College of pharmacy in assisting in handling and management of experimental animals.
References:
1. Tripathi KD. Essentials of medical pharmacology. Jaypee brothers medical publishers (p) ltd. Vth Ed. New Delhi; 2004. p. 167-181, 257-259
2. Harris EDJR (1990).Rheumatoid arthritis: pathophysiology and implications for therapy. N.Engl. J. Med. 322: 1277-1289.
3. Scott DL, Shipley M, Dawson A, Edwards S, Symmons DP, Woolf AD (1998). The clinical management of rheumatoid arthritis and osteoarthritis: strategies for improving clinical effectiveness. Br. J. Rheumatol. 37: 546–554.
4. CSIR. 1997. MEDICINAL PLANTS. Their bioactivity, screening and evaluation. Proceedings of the international workshop, held at Lucknow (India), organized by Center for Science and Technology of the nonaligned and other developing countries with the support of UNIDO and CSIR.
5. Kritikar KR and Basu BD. Indian Medicinal plants. 2nd Edition, International Book distributor, Dehradun, India. 1935. pp 2011- 2012.
6. Nadkarni KM. Indian materia medica. 3rd Edition, Popular Prakashan Pvt Ltd, Mumbai. 1996. pp 114-115.
7. Perumalsamy R, Maung Thwin M, Gopalakrishnakone P and Ignacimuthu S, Ethno-botanical survey of folk plants for the treatment of snakebites in southern part of Tamilnadu, India. Journal of Ethnopharmacology. 115 (2): 2008. 302-312.
8. Kalyani K, Lakshmanan KK and Viswanathan MB, Medico-botanical Survey of plants in Marudhamalai Hills of Coimbatore district, Tamilnadu. Journal of the Swamy Botanical Club. 6 (3 and 4): 1989. 89-96.
9. Ganesan S. Traditional oral care medicinal plants survey of Tamilnadu. Natural Product Radiance. 7 (2) : 2008. 166-172.
10. Brindha P, Rukmani B and Purushothaman KK. Pharmacognostic studies on Anisomeles malabarica R. Br. Bulletin of Medico-ethnoBotonical Research. 4: 1977. 74-84.
11. Mukherjee PK. Quality Control of Herbal Drugs, Business Horizons Pharmaceutical Publishers, New Delhi, 2010, pp. 184-191.
12. Kokate CK, Khandelwal KR, Pawar AP and Gohale SB. Practical Pharmacognosy, Nirali Prakashan, Pune, India. c1995. pp 137-139.
13. Turner RA. Screening methods in pharmacology, Academic Press, London, 61, 1965.
14. Kulkarni SK. Handbook of Experimental Pharmacology, 2nd edition, 78-81, 1993
15. Ravi V, Saleem TSM, Patel SS, Raamamurthy J, Gauthaman K, AntiInflammatory Effect of Methanolic Extract of Solanum nigrum Linn Berries. International Journal of Applied Research in Natural Products, 2(2), pp. 33-36, 2009.
16. Winter CA., Risley EA and Nuss GW. Carrageenan induced oedema in hind paw of the rats as an assay for anti-inflammatory drugs. Proceedings of the Society for Experimental Biology and Medicine. 11: 1962. 544-547.
17. Seibert K, Zhang Y, Leahy K and et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci 1994; 91: 12013–12017.
18. Vinegar R, Schreiber W, Hugo R. Biphasic development of carrageenan edema in rats. J Pharmacol Exp Ther 1969; 166: 96–103.
19. Crunkhorn P, Meacock SC. Mediators of the inflammation induced in the rat paw by carrageenan. Br J Pharmacol 1971; 42: 392– 402.
20. Di Rosa M, Willoughby DA, Screens for anti-inflammatory drugs. J Pharm Pharmacol 1971; 23: 297–298.
21. Hirasawa N, Watanabe M, Mue S, Tsurufuji S, Ohuchi K. Downward regulation of neutrophil infiltration by endogenous histamine without affecting vascular permeability responses in air pouch type carrageenan inflammation in rats. J Inflammation 1991; 15: 117– 126.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License