IJCRR - 4(14), July, 2012
Pages: 29-37
Date of Publication: 31-Jul-2012
Print Article
Download XML Download PDF
STUDIES ON FUNGAL DECOLOURIZATION OF SYNTHETIC DYES
Author: D.Sudha, R.Balagurunathan
Category: General Sciences
Abstract:Decolourization of synthetic dyes like direct greenish blue, direct brilliant violet using Aspergillus niger and Phanerochaete Chrysosporium was carried out at a dye concentration of about 10mg/l. Asthana and Hawker's broth was used for decolourization study 10mg/l of two dyes were added and mixed well. After that, the flasks were inoculated with pre-grown fungal mycelial cultures. During the incubation, samples were drawn at 1, 3, 7 and 14 days and analyzed for decolourization, after centrifuging the samples at 8000-9000rpm for 15 minutes. The Supernatent was collected and absorbance was measured using Spectronic 20 at 490nm. The maximum decolourization of direct brilliant violet of about 65%,60% were achieved by Aspergillus niger and Phanerochaete Chrysosporium respectively during 14th day of incubation and the maximum decolourization of direct greenish blue about 76%,70% were achieved by Aspergillus niger and Phanerochaete Chrysosporium respectively during 14th day of incubation. The maximum percentage of degradation of direct brilliant violet was 65% and direct greenish blue was 76% obtained from Aspergillus niger. In order to treat the dyes more effectively, microorganism capable of degrading the toxic compound present in the textile dyes will be used on a large scale and will be introduced into effluent treatment plants for Bioremediation. Keywords: Fungal isolates, Synthetic dyes, Decolourization, Aspergillus niger and Phanerochaete Chrysosporium. ______________
Keywords: Fungal isolates, Synthetic dyes, Decolourization, Aspergillus niger and Phanerochaete Chrysosporium.
Full Text:
INTRODUCTION
Azo dyes are the largest group of dyes used in industry (Ramalho, et al., 2002; Mane, et al., 2008) representing more than half of the annual production (Stolz, 2001). Technological advance has seen an increase in diversity and complexity of synthesized textile dyes with the objective of product improvement through enhancement of dye properties such as resistance to fading, improved delivery of dyes to fabrics and increased variety of shades. This increase in diversity and complexity of dyes is coupled with higher resistance to environmental degradation leading to pollution problems by textile effluent. A larger proportion of these azo dyes which can pass through normal water treatment procedures (Stolz, 2001; Pearce, et al., 2003; Pandey, et al., 2007; Chamunorwa Aloius Togo, 2008) resulting in aesthetically unappealing water. Due to the fungal oxidative mechanisms, it is possible to avoid the formation of hazardous anilines, formed by reductive cleavage of the azo dyes. Compared to other fungal oxidative enzymes, laccases can act oxidatively, non specifically at the aromatic rings and has the potential to degrade a wide range of compounds. Laccases (benzenediol: oxygen oxidoreductase, EC 1.10.3.2) are multicopper containing enzymes that catalyze the oxidation of a variety of phenolic and inorganic compounds, with the concomitant reduction of oxygen to water. Due to their wide substrate specificity, laccases have gained much attention over the last number of years in many industrial and environmental fields (Fernandes et al., 2008; Moldes et al., 2008; Sadhasivam et al., 2009). Enzymatic processes have various advantages over conventional, biological, physical, and chemical treatment processes including selective removal of particular pollutants, application to xenobiotic recalcitrant compounds, high reaction rate and reduction in sludge volume (Sadhasivam, 2007). In recent years, dye decolorization by laccases has received a significant attraction by various researchers (Domi‘nguez et al., 2005; Moldes and Sanroman, 2006). Adequate treatment of textile effluent requires more than one stage as there is need for both colour removal and degradation of aromatic compounds from the decolourization process. Physico-chemical treatment methods are the least desirable owing to their high costs and generation of secondary pollutants. On the other hand, biological treatment methods are attractive due to their cost effectiveness, diverse metabolic pathways and versatility of microorganisms (Singh, et al., 2004; Van de Zee and Villaverde, 2005).
MATERIALS AND METHODS
Dyes and soil sample collection Dyes used in the experiments were purchased from Infra-Tex dye industry, Perunthurai, in Tirupur and were of the highest purity. The soil sample was collected from dye contaminated area nearby textile industry situated in Perunthurai (Erode District), Tamilnadu, India.
Decolourization media
Asthana Hawker‘s broth (Laxminarayana, et al., 2010) was composed of (5 gm glucose, 3.5 g KNO3 1.75 g KH2PO4, 0.75 g MgSO4 7H2O and I L distilled water) was prepared and added with 10mg dye the medium was sterilized at 121°C for 20 minutes and used for the cultivation of mycelia and for decolorization experiments.
Isolation techniques
The soil sample was serially diluted in sterile physiological saline and was transformed to sabouraud‘s dextrose agar. The plates were incubated at room temperature for 3 days. After incubation the plates were noted for the presence of fungal colonies (Cappucino Sherman, 2007).
Identification of fungal isolates- lacto phenol cotton blue staining- tease mount preparation
A drop of lacto phenol cotton blue was placed on a clean glass microscopic slide. With a straight wire slightly bended at the tip, a small portion of the colony was removed and placed it in the drop of LPCB. With the help of another straight wire (or) needle the fungal culture tint was teased into small bits and spread in LPCB. Cover slip was placed and pressure was gently applied over the agar bits to spread evenly. After giving sufficient time for the structure to take up the stain, the slide was examined using microscope under 10x, 45x objectives to examine the fungal morphology. Based on morphological and cultural characteristics, the isolated fungal cultures were identified as Aspergillus niger, Phanerochaete chrysosporium (Cappucino Sherman, 2007).
Decolourization
Asthana and Hawker‘s broth was used for decolourization study 20ml broth was taken in 100ml Erlenmeyer flask and sterilized. Then 10mg/l (Dilek Asma, et al., 2006) of direct brilliant violet and direct greenish blue were added and mixed well. After that, the flasks were inoculated with pre-grown fungal mycelial cultures.
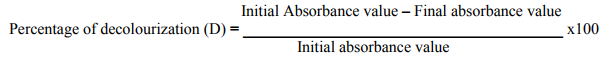
During the incubation, samples were drawn at 1, 3, 7 and 14 days and analyzed for decolourization, after centrifuging the samples at 8000-9000rpm for 15 minutes. The supernatant was collected and absorbance was measured using spectronic 20 at 490nm. The percentage of decolourization was calculated using the formula given below Decolourizing activity was expressed in terms of percent decolourization (Yatome et al., 1993).
Scanning of dyes for its absorbance maximum
The maximum absorbance of the dyes was scanned using spectronic 20 from 490nm. The dyes direct brilliant violet and direct greenish blue, gave a maximum absorbance at 490nm (Soeprijanto et al., 2002) respectively and the samples were analyzed at their respective absorbance throughout the study.
Biomass Estimation
The fungal biomass (pellets) produced was filtered out from the liquid medium by using Whatman filter paper No. 1. It was washed twice with deionized water before application in the experiments. The fungal biomass dried at 110?C for 5 hrs in hot air oven and the dry weight was measured (Ashu – Augustine, et al., 2006). p H of the medium p H of the supernatant was measured using the pH meter during initial stage and 1, 3, 7 and 14 days of incubation in the case of commercial dyes.
RESULTS
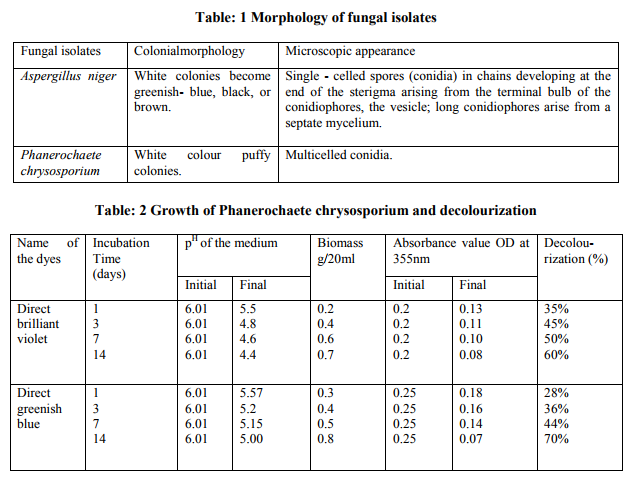
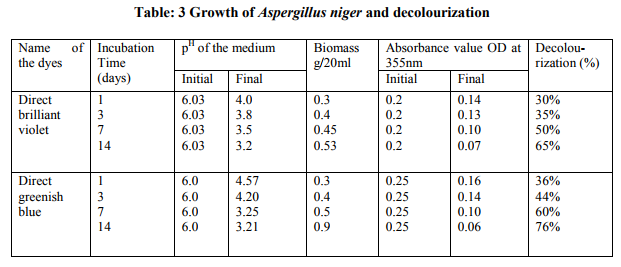
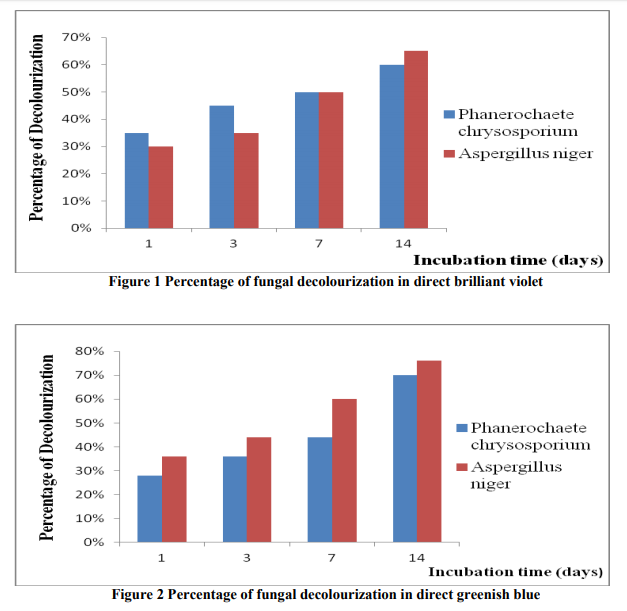
The decolourization studies carried out with fungal isolates from the dye effluent Based on morphological and cultural characteristics, the isolated fungal cultures were identified as Aspergillus niger, Phanerochaete chrysosporium (table-1). The results shows that Aspergillus niger performed best when compared to Phanerochaete chrysosporium (tables 2, 3 and figures 1, 2). The synthetic dyes removal was tested using two approaches. The first one was based on the biomass formation the second one was based on p H . Samples were withdrawn at different intervals after dye amendment until 14th day of incubation to determine the dye bio removal and growth media pH changes. The results showed that Aspergillus niger strain gave good efficiency in the removal of direct brilliant violet in 14th day of incubation, where the decolourization reached to 76% (figure). The same fungal strain gave 65% percentage of decolourization in direct greenish blue at 14th day of incubation. Another fungal isolute Phanerochaete chrysosporium gave 70% in direct greenish blue than the direct brilliant violet (60%), on 14th day of incubation. In, the second approach the dye removal in the pH 3.0 – 6.0 showed biomass production of maximmum 0.9g / 20ml by Aspergillus niger at pH 3.0, and 0.8 g / 20 ml by Phanerochaete chrysosporium at pH 5.0. Fungal growth did not occur at pH 2 and 9.
DISCUSSION
Direct greenish blue resulted with maximum decolourization of about 76% in Aspergillus niger 70% in Phaenerochaete chrysosporium. As the decolourization was proceded, the concentration of the dye decreased in terms of its absorbance value at the initial stage, dye decolourization was probably due to the absorption of dye to the mycelium (Cripps, et al., 1990). Likewise, few other studies have also clearly mentioned biosorption/ bioadsorption of certain brown rot Fungi Aspergillus niger, Aspergillus foetidus (Ali, et al., 2007). The primary dyes removal phenomenon coupled with electrostatic pull between the positively charged cell wall and negatively charge dyes (Aksu et al., 1999; Aksu and Tezer, 2000). In this work, the use of Aspergillus niger to remove direct dyes commonly used in textile industry revealed that this fungal culture was capable to remove dyes in short time from the media. This interaction could be based on a biosorption of dyes on the intact fungal biomass; this is in harmony with Juliana and Thuy, 2002. Brilliant green is much more decolorized by Phanerochaete chrysosporium than the structurally similar crystal violet (Paszczynski, A and Crawford, R.L, 1991). Presumably, these differences are duepartly to electron distribution and charge density, although steric factors may also contribute. There appears to have been only one systematic study relating dye structure to degradation by a white rot fungus (Phanerochaete chrysosporium). Pasti-Grigsby, et al (1992) showed that the nature and position of substituents on one of the aromatic rings of azo dyes can markedly influence decolorization, although a simple, clearpattern was not established. In the present study Phanerochaete chrysosporium showed lesser activity when compared Aspergillus niger. Among the two fungal strains tested we found strain, Aspergillus niger which had better potential for the dye decolourization than strain Phanerochaete chrysosporium. White-rot fungi were the fastest decolourizers, but additionally we found very effective decolourizers among brown-rot fungi. The decrease of pH at the end of these experiments may be referred to the excretion of the organic acid by the fungus itself (Abdel – Aal et al., 2001 and Naima et al., 2007). In the present study we also found that the decrease in pH at the end of experiment. Considering the above result we assumed that dye was probably associatd with fungal growth and hyphal uptake mechanism (biosorption / bioadsorption). Textile industries discharge colored dyes and toxic compound in to the environment. Physicochemical methods that are have operation problem and do not provide satisfactory results. But biological treatment methods are cheap and offer the best alternative with proper analysis and environmental control. In order to treat the dyes more effectively, Fungal isolates capable of specifically degrading the toxic compound present in the textile dyes are cultured on a large scale and introduced into effluent treatment plants.
CONCLUSION
The present study reveals that the fungal culture can be used successfully for decolourizing direct brilliant violet and direct greenish blue. 80% of the decolourization of the above dyes were achieved by Aspergillus niger. On the basis of the result of the present study suitable strategy can be developed for the treatment of waste water contaminated with dye. These biological methods can be promoted to degrade the variety of dyes from the textile industries. The treated textile dyes when disposed to the land it has several applications. *improves soil fertility. *very little quantity of bioearth, compost is sufficient for crops and thus input and transport cost could be reduced. *Humus rich, very slow release of nutrient which is essential for growth. *Increases water holding capacity of the soil.
ACKNOWLEDGEMENT
The authors express genuine thanks to the ViceChancellor and the Registrar, Periyar University, Salem, for providing research facilities. Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Abdel – Aal S E, Dessouki A M, Gad Y H. Removal of some dyes from industrial effluents by polymeric materials 7 gamma – irradiation. Journal of Radioanalytical and Nuclear Chemistry 2001; 247 (2): 399 – 405.
2. Aksu Z, Calik A, Dursun AY, Demircans Z. Biosorption of iron (III)-cyanide complex anions to Rhizopus arrhizus: application of adsorption isotherms. Process Biochem 1999; 34: 483-491.
3. Aksu Z, Tezer S. Equilibrium and kinetic modelling of biosorption of Remazol Black B by Rhizopus arrhizus in a batch system: effect of temperature. Process Biochem 2000;36: 431-439.
4. Ali N, Hameed A, Ahmed S, Khan AG. Decolorization of structurally different textile dyes by Aspergillus niger SA1. World J.Microbiol. Biotechnol 2007; 24: 1067-1072.
5. Ashu – Augustine, Imelda – Joseph and Paul Raj R. Biomass estimation of Aspergillus niger s14 a mangrove fungal isolate and Aspergillus Oryzae NCIM 1212in solid state fermentation, J. Mar. Biol. Ass. India 2006; 48 (2): 139-146.
6. Cappucino Sherman. Microbiology a Laboratery Manual (Seventh edition) (1st impression), Dorling Kindersley (India) Pvt Ltd 2007; 239 -242.
7. Chamunorwa Aloius Togo, Cecil Clifford Zvandada Mutambanengwe and Christopher George Whiteley. Decolourization and degradation of textile dyes using a sulphate reducing bacteria (SRB) – biodigester microflora co-culture. African Journal of Biotechnology 2008; 7 (2): 114-121.
8. Cripps C, Bumps JA and Aust D. Biodegradation of azo and heterocyclic dyes by Phanerochaete chrysosporium. Appl.Environ.Microbiol 1990; 56: 1114-1118.
9. Dilek Asma, Sibel Kahraman, Seval Cing and Ozfer Yesilada. Adsorptive removal of textile dyes from aqueous solutions of dead fungal biomass. Journal of Basic Microbiol 2006; 46(1): 3-9.
10. Dom?‘nguez A, Rodr?‘guez Couto S, Sanroman MA. Dye decolorization by Trametes hirsuta immobilized into alginate beads. World J Microbiol Biotechnol 2005; 21: 405–409.
11. Fernandes SC, Oliveira IRWZ, Fatibello-Filho O, Spinelli A, Vieira IC. Biosensor based on laccase immobilized on microspheres of chitosan crosslinked with tripolyphosphate. Sens Actuators B Chem 2008; 133: 202–207.
12. Juliana, A.R and Thuy N. Decolourization of textile dyes Trametes versicolor and its effect on dye toxicity. Biotechnology Letters 2002; 24: 1757-1761.
13. Laxminarayana E, Thirumala Chary M, Randheer Kumar M and Singara Charya M. A Decolourization and biodegradation of sulphonated azo dyes by fungi to clean dye contaminated soil environments, Journal of Natural and Environment Sciences 2010; 1(1): 35-42.
14. Mane, U. V., Gurav, P. N., Deshmukh, A. M., Govindwar, S. P.. Degradation of textile dye reactive navy – blue Rx (Reactive blue–59) by an isolated actinomycete Streptomyces krainskii SUK – 5, Malaysian Journal of Microbiology 2008; 4(2): 1-5.
15. Moldes D, D?´az M, Tzanov T, Vidal T. Comparative study of the efficiency of synthetic and natural mediators in laccase assisted bleaching of eucalyptus kraft pulp. Bioresour Technol 2008: 99:7959–7965.
16. Moldes D, Sanroman MA. Amelioration of the ability to decolorize dyes by laccase: relationship between redox mediators and laccase isoenzymes in Trametes versicolor. World J Microbiol Biotechnol 2006; 22:1197– 1204.
17. Naima C, Delia – Laura P, Douglas A M, Arani C, Deiter L, Alexander D R, Karl Werner S and Terrence J C. Fe111 – TAML – catalyzed green oxidative degradation of the azo dye orange II by H2O2 and organic peroxides: products, toxicity, kinetics and mechanism. Green Chem 2007, 9: 49 –57.
18. Pandey A, Singh P, Iyengar L. Bacterial decolourization and degradation of azo dyes. Int. Biodeterior. Biodegrad. 2007; 59: 73-84.
19. Paszczynski, A. and Crawford, R. L. Degradation of azo compounds by ligninase from Phanerochaete chrysosporium: Involvement of veratryl alcohol. Biochem. Biophys. Res. Commun., 1991; 178: 1056- 1063.
20. Pasti-Grigsby, M. B., Paszczynski, A., Goszczynski, S., Crawford,D. L. and Crawford, R. L. Influence of aromatic substitution patterns on azo dye degradability by Streptomyces spp. and Phanerochaete chrysosporium. Appl. Environ. Microbial 1992; 58: 3605-3613.
21. Pearce CI, Lloyd JR, Guthrie JT. The removal of colour from textile waste water using whole bacterial cells: a review. Dyes Pigm. 2003; 58: 179-196.
22. Ramalho, P. A., Scholze, H., Cardoso, M. H. Ramalho, M. T. Oliverira and Campos, A. M. Improved conditions for the aerobic reductive decolourization of azo dyes by Candida zeylamoides, Enzyme and Microbial Technology 2002; 7: 402 – 412.
23. Sadhasivam S. Production, characterization and application of laccase from the ascomycetous fungus Trichoderma harzianum and utilization of biomass produced as biosorbent for colour removal, Ph.D., thesis 2007, Department of Biotechnology, Bharathiar University, Coimbatore, India.
24. Sadhasivam S, Savitha S, Swaminathan K. Redox-mediated decolorization of recalcitrant textile dyes by Trichoderma harzianum WL1 laccase, World J Microbiol Biotechnol 2009; 25:1733–1741.
25. Singh P, Mishra LC, Iyengar L. Biodegradation of 4- aminobenzene sulphonate by newly isolated bacterial strain PNS-1. World J. Microbiol. Biotechnol. 2004; 20: 845-849.
26. Stolz, A. Basic and applied aspects in the microbial degradation of azo dyes. Applied Microbiology and Biotechnology 2001; 56: 69 – 80.
27. Soeprijanto, Ivan, P., dody, A. A., and Larsen, V. F, Decolourization of synthetic dye using white rot fungi in a rotary biological contactor. Asian Pacific Confederation of Chemical Engineering 2002; 280.
28. Van de Zee FP, Villaverde S. Combined anaerobic-aerobic treatment of azo dyes – a short review of bioreactor studies. Water Res. 2005; 39: 1425-1440.
29. Yatome C, Yamada S, Ogawa T and Matsui M. Degradation of crystal violet by Nocardia corallina . Appl Microbiol Biotechnol, 1993; 38: 565-569.

|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License