IJCRR - 4(19), October, 2012
Pages: 181-191
Date of Publication: 15-Oct-2012
Print Article
Download XML Download PDF
BASIC FACTS OF FIRE - A FORENSIC REVIEW
Author: Pragnesh Parmar, Gunvanti B. Rathod
Category: Healthcare
Abstract:Fire is a complex chemical process and one of the most common causes of loss of property and life. Forensic investigator must understand the basic chemistry and physics of fire to know the exact origin and cause of it. The diffusion flame process consists of three basic elements: fuel, oxygen and heat. The six elements of the life cycle of fire are described by Dawson Powell in The Mechanics of Fire. These elements are input heat, fuel, oxygen, proportioning, mixing, and ignition continuity. All fires produce combustion products. Combustion products fall into four categories: heat, gases, flame, and smoke. A fire in a room or defined space generally will progress through three predictable developmental stages. In order to determine the origin and cause of a fire properly, the investigator must be able to interpret the effects of these stages correctly during the examination of the fire scene. In this paper, I discussed about the life cycle of fire, effect of various fire gases, flame colour, flame types and room fire sequence to provide a scientific basis for determination of origin and cause of fire for investigator.
Keywords: Life cycle of fire, Fatality of fire gases, Stages of room fire, Forensic investigator, Flame colour and temperature.
Full Text:
INTRODUCTION
Fire is a complex chemical process and forensic investigators must understand the basic chemical and physical facts of fire behaviour to enable them to formulate opinions based on scientific principles. Sometimes not being able to explain the technical aspects of fire behaviour may prevent an investigator from qualifying as an expert witness in the court of law. Defense lawyer can easily use questions about the chemical and physical elements of fire to effectively discredit a forensic investigator. In such circumstances, basic chemical and physical facts of fire are very useful for forensic investigators in the court of law. Fire (Diffusion Flame Process) Fire is the rapid oxidation of a material in the exothermic chemical process of combustion, releasing heat, light and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition. The diffusion flame process (fire) consists of three basic elements: fuel, oxygen and heat. The diffusion flame process is defined by Richard Tuve in the Principles of Fire Protection Chemistry as "a rapid self-sustaining oxidation process, accompanied by the evolution of heat and light of varying intensities." [1] Fire in its most common form can result in conflagration, which has the potential to cause physical damage through burning. Fire is an important process that affects ecological systems across the globe. The positive effects of fire include stimulating growth and maintaining various ecological systems. Fire has been used by humans for cooking, generating heat, propulsion purposes and signalling. The negative effects of fire include atmospheric pollution, water contamination, soil erosion and hazard to human and animal life. [2]
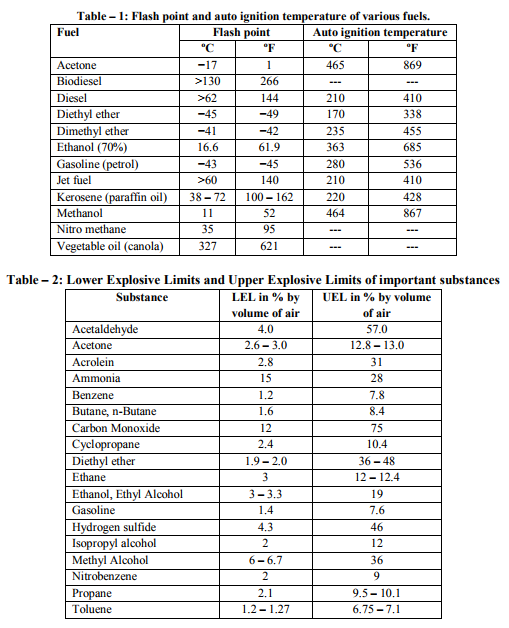
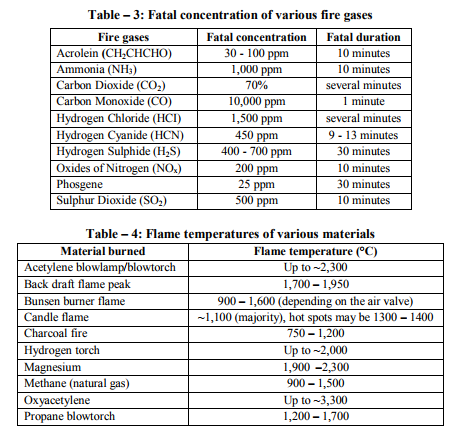
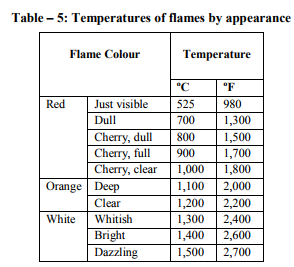
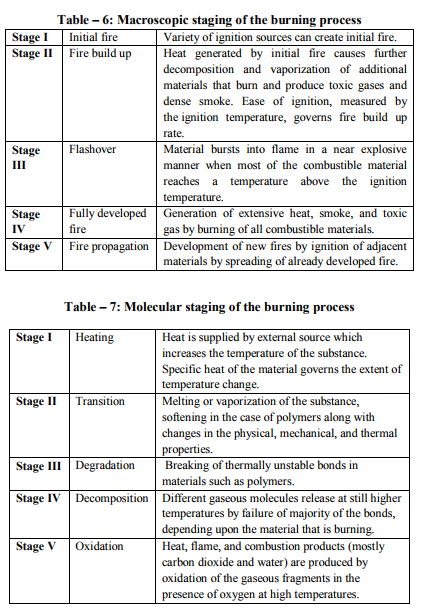
Fires start when a flammable and/or a combustible material, in combination with a sufficient quantity of an oxidizer such as oxygen gas or another oxygen rich compound, is exposed to a source of heat or ambient temperature above the flash point for the fuel/oxidizer mix, and is able to sustain a rate of rapid oxidation that produces a chain reaction. This is commonly called the fire tetrahedron. Fire cannot exist without all of these elements in place and in the right proportions. Some fuel may require a catalyst, a substance that is not directly involved in any chemical reaction during combustion, but which enables the reactants to combust more readily. Fire can be extinguished by removing any one of the elements of the fire tetrahedron. For example, in case of stovetop burner, fire can be extinguished by turning off the gas supply, covering the flame completely, application of water and application of a retardant chemical such as Halon to the flame. [2] Life cycle of fire Dawson Powell in The Mechanics of Fire described 6 basic elements of life cycle of fire. These elements are input heat, fuel, oxygen, proportioning and mixing along with ignition continuity. Both the initiation and continuation of the burning process requires all of these elements. Fire triangle includes first three elements - input heat, fuel, and oxygen, which are familiar to all forensic investigators. [3] Input Heat Vapors are produced by heating of solid and liquid materials, which are actually responsible for burning process. Flashpoint of a volatile material is the lowest temperature at which it can vaporize to form an ignitable mixture in air. Flame point is the temperature at which the fuel will continue to produce sufficient vapors to sustain a continuous flame which is a few degrees above the flash point. Ignition temperature is the temperature at which the vapors will ignite. [3, 4, 5, 6] Flash point and auto ignition temperature of various fuels are given in Table - 1. [7, 8] Fuel Fuel may be in the form of a gas, liquid or solid. Liquid and solid fuels must be heated sufficiently to produce vapors. In general terms, combustible means capable of burning, generally in air under normal conditions of ambient temperature and pressure, while flammable means capable of burning with a flame. Flammable liquids are those which have a flashpoint < 100 °F (37.8 ?C), such as acetone, ethyl alcohol and gasoline. Combustible liquids are those which have a flashpoint > 100 °F (37.8 °C), such as fuel oil and kerosene. The specific gravity of a liquid is the ratio of the weight of the substance to that of water, which is assigned a value of 1. A substance with a specific gravity > 1 will tend to sink, while one whose specific gravity is < 1 will tend to float. [3, 4, 5, 6] Oxygen Oxygen for burning process is derived from the atmosphere, which contains approximately 20.8 % oxygen. Charring or smoldering (pyrolysis) can occur with as little as 8 % oxygen but continuation of flaming combustion requires at least 15 - 16 %. Transformation of a compound into other substances by heat alone is called as pyrolysis. Oxidizers are the chemicals which can be act as primary or secondary source for burning process e.g. ammonium nitrate and chlorine. [3, 4, 5, 6] Mixing and Proportioning Mixing and proportioning are continuous reactions which must be required for fire to propagate. Correct proportions of fuel vapors and oxygen are must for fire, within the explosive limits or flammable limits. Explosive or flammable limits are expressed in the concentration (percentage) of fuel vapors in air. A mixture, containing fuel vapors, in an amount less than necessary for ignition to occur is too lean, while a mixture, containing too high concentration of fuel vapors is too rich. Lower Explosive Limit (LEL) is the lowest concentration that will burn, while the Upper Explosive Limit (UEL) is the highest concentration that will burn. [9, 12] Lower Explosive Limit and Upper Explosive Limit of various important substances are given in Table – 2. [10] Vapor density is the weight of a volume of a given gas to an equal volume of dry air, where air is given a value of 1.0. A vapor density of < 1.0 means that the gas is lighter than air and will tend to rise in a relatively calm atmosphere, while a vapor density of > 1.0 means that the gas is heavier than air and will tend to sink to ground level. [4, 5, 6] Ignition Continuity Thermal feedback from the fire to the fuel represents ignition continuity. Heat is transferred by conduction, convection, radiation and direct flame contact. Conduction means transfer of heat by direct contact. Convection means transfer of heat by changes in density of liquids and gases. Radiation means the transfer of heat by infrared radiation which generally is not visible to the naked eye. Direct flame contact is a combination of two of the basic methods of heat transfer. [4, 11] The amount of heat generated is measured in British thermal units. 1 British thermal unit is the amount of heat required to raise the temperature of 1 pound of water to 1 °F when the measurement is performed at 60 °F (15.5 °C). [1, 4]. Depending upon burning of material, fire can be classified into 4 basic types. Class A fires involve ordinary combustible materials, such as cloth, paper, plastics, rubber, wood etc. Class B fires involve flammable or combustible liquids, greases and gases. Class C fires involve energized electrical equipment like short circuiting machinery, overloaded electrical cables etc. Class D fires involve combustible metals such as magnesium, potassium, sodium, titanium and zirconium. [11, 13]. Combustion products (heat, gases, flame, and smoke) are produced by all type of fires. Heat is defined as a form of energy characterized by vibration of molecules and capable of initiating and supporting chemical changes and changes of state. Gases are originally shapeless and volume less substances which expand to take the shape and volume of the space they occupy. Fire gases include acrolein, ammonia, carbon monoxide, hydrogen cyanide, hydrogen chloride etc. Flame is the luminous portion of burning gases or vapors. Smoke is the airborne particulate products of incomplete combustion, suspended in gases, vapors, or solid or liquid aerosols containing soot, black particles of carbon. [11, 12] Fatal concentration of various fire gases are given in Table – 3. [14]. Stages of Room Fire Room fire is usually progress through 3 developmental stages. To determine the origin and cause of a fire, forensic investigator must be able to interpret these stages correctly during the examination of the fire scene. [4] First stage of fire development is incipient stage (growth). This stage begins at the moment of ignition when flames are localized and fire is fuel regulated. Fire propagation is regulated not by the available oxygen but by the configuration, mass and geometry of the fuel itself. The oxygen content is within the normal range and normal ambient temperatures still exist. Hot fire gases will begin to rise to the upper portions of the room and draw additional oxygen into the bottom of the flames. Fire gases such as carbon monoxide, sulphur dioxide etc. will begin to accumulate in the room. [4, 9] Second stage is free burning stage (development). In this stage, more fuel is being consumed, fire is intensifying; flames are spreading upward and outward by convection, conduction and direct flame impingement. A hot, dense layer of smoke and fire gases is collecting at the upper levels of the room, radiating heat downward, containing not only soot, but also toxic gases such as acrolein, carbon monoxide, hydrogen cyanide, hydrogen chloride etc. The temperature at the ceiling level has begun to rise rapidly while the floor temperature is still relatively cool. It is still possible to survive in the room at the cooler lower level. [4, 9, 15] When ignition of the upper layer results in fire, extending across the room at the ceiling level is called Rollover. This rollover causes the ceiling temperature to increase at greater rate and also increases the heat being radiated downward into the room. The fire is still fuel regulated at this time. [15] Flashover occurs when the upper layer reaches a temperature of approximately 1,100 °F (593.3 °C) and sufficient heat is generated to cause simultaneous ignition of all fuels in the room. Survival for more than a few seconds is impossible after flashover has occurred. Temperatures in the space will reach > 2,000 °F (1,093.3 °C) at the ceiling level and 1,000 °F (593.3 °C) at the floor. At the point of flashover the fire is still fuel regulated, however, if the fire stays confined to the room of origin it quickly becomes oxygen regulated. The rapid temperature rise associated with flashover generally results in windows breaking, which then produces an unlimited supply of oxygen causing the fire to transfer back to the fuel regulated phase. [15] Flashover results in intense burning of the entire room and its contents. Fuel package, the room geometry and ventilation affect the length of time necessary for a fire to go from the incipient stage to flashover. In the typical residential accidental fire setting, this time may be as short as 2 - 3 minutes. [15] Third stage is smoldering stage (decay). In this stage, the fuel is consumed and open burning becomes increasingly less prevalent. Open flaming combustion will stop if the fire has been contained to a room or space, and the oxygen level drops < 15 - 16 %, even if unburned fuel is still present. Glowing combustion will take place at this point. High temperatures and considerable quantities of soot and combustible fire gases have accumulated, and at this point the fire is oxygen regulated. The temperatures may exceed the ignition temperatures of the accumulated gases. The accumulated soot and fire gases may ignite with explosive force if a source of oxygen is introduced in the area. This smoke explosion is known as a back draft. The pressures generated by a back draft are enough to cause significant structural damage and endanger the lives of persons and bystanders. Back drafts can take place in any enclosed space; they are not limited to rooms. Attics, basements, and concealed ceiling spaces also are susceptible. [12, 13] Flame Types A flame (from Latin flamma) is the visible (light emitting), gaseous part of a fire. If hot enough, the gases may become ionized to produce plasma. [2] Depending on the substances alight, and any impurities outside, the colour of the flame and the fire's intensity will be different. Flames can be divided into 4 categories: laminar premixed, laminar diffusion, turbulent premixed and turbulent diffusion. An example of a laminar premixed flame is a Bunsen burner flame. Laminar means that the flow streamlines are smooth and do not bounce around significantly. Premixed means that the fuel and the oxidizer are mixed before the combustion zone occurs. A laminar diffusion flame is a candle. The fuel comes from the wax vapor, while the oxidizer is air; they do not mix before being introduced (by diffusion) into the flame zone. A peak temperature of around 1400 °C is found in a candle flame. [16] Most turbulent premixed flames are from engineered combustion systems: boilers, furnaces, etc. In such systems, the air and the fuel are premixed in some burner device. Most unwanted fires fall into the category of turbulent diffusion flames. Since no burner or other mechanical device exists for mixing fuel and air, the flames are diffusion type. [17] Flame temperature of various materials is given in Table – 4. [18] Flame Colour Flames have many different colours that range from blue to red to orange to yellow. It's even possible to have green flames, although that's not real common in campfires and other small fires that most folks are familiar with. [19] The hotter the temperature in a flame, the bluer the color will be. The lowest temperatures in a flame result in redder colors. When you look at a flame, the blue colors will occur near the combustion site, or nearest what is being burned. For example, when a candle is burned, the color nearest the wick will be blue, usually light blue. As you look at the flame farther from the wick, where the temperature is lower, you will see oranges, then yellows and possibly reds.[20] Colour tells us about the temperature of a candle flame. The inner core of the candle flame is light blue, with a temperature of around 1670 K (1400 °C). That is the hottest part of the flame. The colour inside the flame becomes yellow, orange, and finally red. The further you reach from the centre of the flame, the lower the temperature will be. The red portion is around 1070 K (800 °C). [21]Temperature of flames by appearance is given in Table – 5. [2] Stages of the burning process Macroscopic staging of burning process is given in Table – 6 and molecular staging of burning process is given in Table – 7. [22] Fire and human body In case of direct contact with the flames, the organic matter of the body is consumed as fuel. Above 51°C, the excess heat is no longer conducted away by convection via the capillaries of the skin. Penetrating power of moist heat is considerably higher than that of dry heat. Effects of heat on the body and related external and internal findings are given in Table – 8. [23] Classification of the destruction by burns according to Eckert et al is given in Table – 9. [24] Classification of the destruction by burns according to Maxeiner is given in Table -10. [25] Crow - Glassman Scale (CGS) of burn related destruction of corpses is given in Table -11. [26] Classification of the destruction by burns according to Gerling et al. is given in Table -12. [27]
CONCLUSION
Fire is a very complex process influenced by many factors that affect its growth, spread and development. The physical shape and state of the fuel, the available oxygen and the transmission of heat all play vital roles in fire development. While each fire is different, all fires follow certain predictable patterns which, when understood by the investigator, provide a scientific basis for determination of origin and cause.
ACKNOWLEDGEMENT
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors /publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Tuve, Richard L. Principles of Fire Protection Chemistry. Quincy: National Fire Protection Association, 1976.
2. Law C. K., Laminar premixed flames - Combustion physics, Cambridge University Press, 2006, Page - 300.
3. Powell, Dawson. The Mechanics of Fire. Rhode Island: Grinnell Company Inc., 1955.
4. DeHaan, JohnD, Kirk's Fire Investigation, 3 rd edition, New Jersey: Brady, 1991.
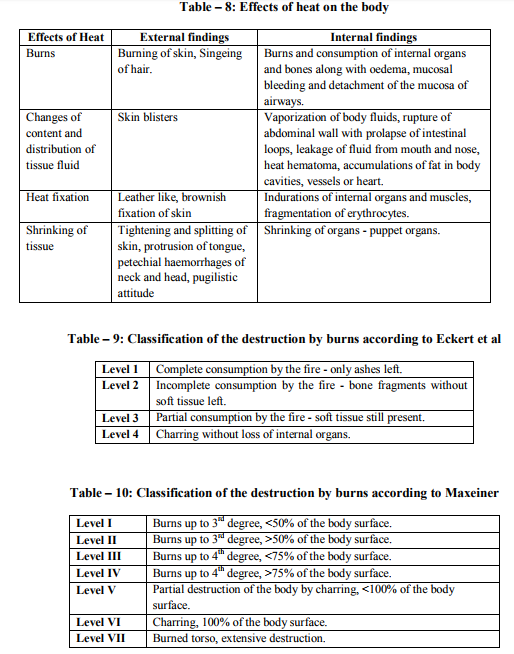
5. Drysdale, Dougal, An Introduction to Fire Dynamics, New York: Wiley, 1986.
6. Fire Dynamics. Emmitsburg: National Fire Academy, 1984. http://www.maiif.org/pdf/fire_chapter1.pdf. Assessed on 10-12-2011.
7. Jozef Jarosinski, Bernard Veyssiere, Combustion phenomena: Selected mechanisms of flame formation, propagation and extinction, CRC Press, 2009, Page – 172.
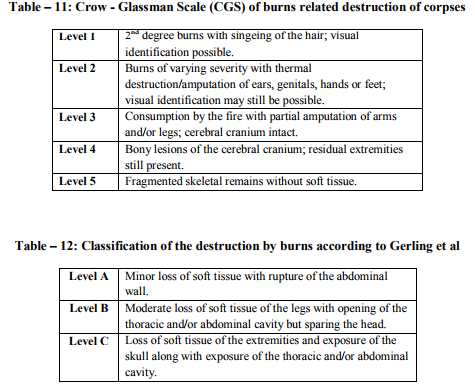
8. Christopher W. Schmidt, Steve A. Symes, The analysis of burned human remains, Academic Press, 2008, Page 2–4.
9. Fang, J.B. Fire Development in Residential Basement Rooms. National Institute of Standards and Technology. http://www.fire.nist.gov/bfrlpubs/fire80/PDF/f 80008.pdf. Assessed on 10-12-2011.
10. Kirshenbaum A. D. and A. V. Grosse, The combustion of Carbon Subnitride, NC4N, and a chemical method for the production of continuous temperatures in the range of 5000– 6000°K, Journal of the American Chemical Society, May 1956, 78(9): 2020.
11. Fire Protection Handbook, 17th edition, Quincy: National Fire Protection Association, 1991.
12. Fung, Francis. "The NBS Program on Corridor Fires" Fire Journal, May 1973. http://fliiby.com/file/34316/goo3s3itjy.html. Assessed on 10-12-2011.
13. NFPA 921, Guide for Fire and Explosion Investigations, 1992 edition, Quincy: National Fire Protection Association.
14. NFPA 325M, Manual on Fire Hazard Properties of Flammable Liquids, Gases, and Volatile Solids. 1991 ed. Quincy: National Fire Protection Association.
15. NFPA 1033, Standard for Professional Qualifications for Fire Investigator. Quincy: National Fire Protection Association, 1993.
16. Gaydon A. G. and Wolfhard H. G., Flames: Their Structure, Radiation and Temperature, 3 rd edition, Chapman and Hall, London, 1970.
17. Dr. Vytenis Babrauskas. Temperatures in flames and fires, Fire Science and Technology Inc., 2006. 18. Thomas N., Gaydon A.G., Brewer L., et al., Cyanogen Flames and the Dissociation Energy of N2, The Journal of Chemical Physics, 1952, 20(3): 369–374.
19. Jones, John Clifford, Low temperature oxidation and hydrocarbon process safety: A text for students and professionals, Tulsa, Penn Well, 2003, Page 32–33.
20. Scott C., Glasspool J., The diversification of paleozoic fire systems and fluctuations in atmospheric oxygen concentration, National Academy of Sciences of the United States of America, July 2006, 103(29): 10861–10865.
21. Bowman DM, Balch JK, Artaxo P., et al., Fire in the earth system, Science, 2009, 324(5926): 481–484.
22. Lentile Leigh B., Holden Zachary A., Smith Alistair M. S., Remote sensing techniques to assess active fire characteristics and post fire effects, International Journal of Wild land Fire, 2006, 3(15): 319-345.
23. Michael Tsokos, Forensic Pathology Reviews, Volume 1, Humana Press, 2004, Page 3-13.
24. Eckert WG, James S, Katchis S, Investigation of cremations and severely burned bodies, American Journal of Forensic Medicine and Pathology, 1988, 9: 188–200.
25. Maxeiner H, Umstände und Befunde bei 202 Brandtodesfällen, Beitrage zur Gerichtlichen Medizin, 1988, 46: 313–325.
26. Glassman DN, Crow RM, Standardization model for describing the extent of burn injury to human remains, Journal of Forensic Sciences, 1996, 41: 152–154.
27. Gerling I, Meissner C, Reiter A, Oehmichen M, Death from thermal effects and burns, Forensic Science International, 2000, 115: 33– 41.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License