IJCRR - 4(19), October, 2012
Pages: 92-99
Date of Publication: 15-Oct-2012
Print Article
Download XML Download PDF
BIOLOGICAL AND PRE-CLINICAL TRIAL EVALUATION OF A LOCAL MEDICINAL PLANT BACOPA MONNIERI (L.) PENN
Author: A.K. Azad, M. Awang, M.M. Rahman, S.F.U. Akter
Category: Healthcare
Abstract:This study work was carried out to investigate the pharmacological prooperties of 'Bacopa monnieri'. This study reveals that the plant has antibacterial and antimutagenic activities. Thus an attempt was made to reinvestigate aforementioned antimicrobial as well as cytotoxic properties for the bark constituents. Bacopa monnieri (L.) Penn., (root bark and stem bark) have been intended to be accomplished in this study. Review of the biological activities of Bacopa monnieri (L.) Penn. The powdered plant was successively extracted with ether solvent. The antimicrobial investigation showed activity against four bacteria and one fungus.
Keywords: Antibacterial, Antimutagenic, Menorrhoea, decolorized, saponins, chemotaxonomy, cytotoxic, Isolation.
Full Text:
INTRODUCTION
As the civilization progressed the early physicians were guided in great part by these observations [Hill, 1972]. The use of plants for curing various human ailments figured in ancient manuscripts such as the Bible, the Rig Veda (4500-1600 BC), the Elliad and the Odyssey and the history Herodotus [Kochhler, 1981]. The earliest use of medicinal plants in this subcontinent was evaluated by two Indian authors Charaka and Susruta [Chopra et al, 1956]. According to this superstitious doctrine all plants possessed some sign, given with heart- shaped leaves should be used for heart ailments, the sap of blood root (Sanguinaria Canaderisis) as a blood tonic, the liver leaf with its three lobed leaves for liver troubles, the walnut with numerous invaginations and convulsions for brain diseases and pomegranate seeds for dental diseases and so on [Graves, 1990] which are known to have been investigated pharmacologically, out of an estimated 250000 to 500000 species of higher plants growing on earth [Farnsworth, et al 1985]. More than 47% of all drugs, used in Russia, are obtained from botanical sources [Ampofo, 1979]. At present, thousands of plant metabolites are being successfully used in the treatment of variety of diseases [Farnsworth, et al 1985]. A few striking examples of plant metabolites include taxol from Taxus brevifolia [Kumar, et al, 1994], vincristine and vinblastine from Vinca roseus [Satoskar, 1980]. All of which are important anticancer agents being used clinically. In the current popular field of chemotherapy, cepharanthine, isolated from Stephanie cepharantha and Stephania sasaki [Jap. Journ. Exp. Med. 1949, 1:69] is being used as a prophylactic in the management of tuberculosis. Even today 30% of rural population depends on herbal medicine for maintaining its health and well being [Ghani, 1987]. The consumption of medicinal plants is increasing in many developed countries, where 35% of drugs contain active principles from natural origin [Irvine, 1995]. In China, 150CO factories are involved in producing herbal drugs, herbal medicines have been developed to a remarkable standard by applying modern scientific technology in many countries, such as, China, India, Bangladesh, Srilanka, Thailand and United Kingdom. In these countries, the dependence on allopathic drugs has been decreased to greater extent [Borin, 1998]. In 1960, 47% of drugs, prescribed by the physicians in the United States of America, were from natural sources [Binge et al., 1960]. In 1967, 25% of the products, which appeared in 1.05 billion prescriptions filled in the United States contained one or more ingredients derived from higher plants [Korolkovans, et al. 1966]. In the United States, in 1980, the consumers paid 8 billion dollars for prescription drugs in which the active ingredients are still derived from plants [Sofowora 1982] contained as one or more active ingredients, a drug of natural origin (Famsworth, 1966). Modern medicine still has much to learn from the collector of herbs said Dr. Halfdan Mahler, Director General of the World Health Organization. Many of the plants, familiar to the .witch doctor, really do have the healing power that tradition attaches to them. Indeed, the potential of obtaining new drugs from plant sources is so great that thousands of substances of plant origin are being studied for activity against such formidable foes as heart diseases, cancer, and aids [Kirtikar, et al. 1984 and Grover et, al 1980]. From the literature review to was also found that the plant has direct therapeutic effects on piles, dysentery, dyspepsia, diarrhea, vomiting, giddiness, worms, burning of the skin and menorrhea [Champion,R.H.et,al 1982, the Text book of Darmatology,5th edition,vol-3,p963]. The literature survey reveals that the plant has antibacterial and antimutagenic activities.
MATERIALS AND METHOD
Collection and Extraction of the plant material
The barks (root arid stem) of the plant were collected from campus of Jahangirnagar University by taking great care. Then the barks were separated manually from the undesirable materials. The collected and cleaned barks were cut in to small pieces and then dried in the sun and finally dried in a hot air oven at 50-60°C for 48 hours. After complete drying, the entire portion was reduced to coarse powder with the help of a mechanical grinder. Before grinding, the machine was cleaned to avoid contamination. The dried grinded powder was weighed by using a suitable rough balance. Then the powder was stored in a suitable container for extraction purpose. The cutting, drying, grinding and storage processes were performed for both the root and stem barks. The Soxhlet extractor was used for extraction process. The one hundred gram of the dried powder was taken in a porous bag and placed in the Soxhlet chamber. Before placing, the extractor was washed properly and then dried. The powder was soaked with a small portion of the 500 ml ether solvent for some times. Then the rest of the extracting ether was taken in a flask. Upon heating electrically, the vapors were rise trough side arm and condensed in the condenser. The condensed extract was dripped in to the .porous bag containing the plant powder, extracting it by contact. When the level of ether in the chamber was rise to the top of the siphon tube, the liquid contents of the chamber automatically filtered through the powdered containing bag and siphoned into flask. Thus 2000 ml of ether was used successively for four times i.e. 500 ml in each time. Similarly other two solvents ethyl acetate and alcohol were used respectively. The process was performed for the powders of two barks (root bark and stem bark). So there are three extracts obtained for each case, named- ether extract, ethyl acetate extract and alcohol extract.
Decolonization and purification
All the extracts were made free from pigments (decolorized) and other impurities by filtration method. This was performed by passing the extracts vyc-J3 activated charcoal on a filter paper and collected in a beaker. The charcoal, used, was made activated by heating in a hot air at 110°C for one hour before filtration. The filter paper was wetted with solvent involved in filtration process. The decolorized and purified extracts were then completely dried by sanction with the help of water bath at a temperature of 50-60? C and their yield was determined by using the following formula…..
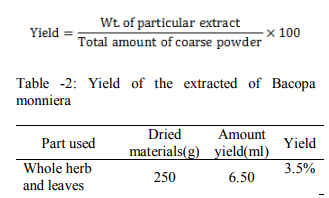
BIOLOGICAL INVESTIGATION
Study of in-vitro antimicrobial activity of the alcohol extracts obtained from the extraction of the bark (root and stem) powder of extracts of Bacopa monnieri (L.). Penn and rectified spirit. The susceptibility of microorganisms to antimicrobial agents could be determined in vitro. The most commonly used method of microbiological assay was the Agar diffusion. In this method, the sample solution was applied on the pail of antibiotic medium and fungus medium seeded with organisms. After proper incubation the sensitivity of the plant extract was evaluated by measuring the diameter of the inhibition zones produced by the samples Nutrient agar is a general culture media which may be used as an enrichment medium by incorporating 10% blood or other biological fluid (Sri vastava O.P 1984). Nutrient agar medium was used for the subculture of the test organisms in which proper growth of the organisms were ensured. From this subculture, a very small amount of sample obtained by harvesting was mixed with the antibiotic medium and fungus medium. The formula and the method of preparation of nutrient agar medium were as follows:

To prepare required volume of this medium, an amount of each of the constituents were calculated from the composition chart given for 1 liter. Peptic digest of animal tissue, sodium chloride, beef extract, yeast extract of required calculated amount were taken in a conical flask by weighing them separately. Distilled water was added to it and the content was heated in a water bath to make a clear solution. The pH was then adjusted to 7.4±0.2 using 10% sodium hydroxide solution. Agar was added to the solution in calculated amount and distilled water was added sufficiently to make a final volume. Again the total volume was heated in water bath to obtain a clear solution. The prepared medium was transferred to each of the required number of bottles which were previously washed. The medium was then sterilized by 'autoclaving for 15 minutes at 121°C under a pressure of I5lb/inch2 . Finally the prepared medium was transferred on to a water bath to reduce the temperature 40-50°C [A.K.Azad et, al, 2012]
Antibiotic Assay Medium, Seed Agar (Hi Media Laboratories’ Ltd., India)
This medium was used for bacterial culture to determine the zone of inhibition. The formula and the method of preparation of this medium were as follows:

Ingredients gm/liter Peptic digest of animal tissue Casein enzymic hydrolysate Yeast extract Beef extract Dextrose Agar 5.00 4.00 3.00 1.40 1.OO 13.00 The required amount of each of the constituents were calculated from the composition chart given for liter and were suspended in required amount of cold distilled water and was boiled for about 15 minutes till complete dissolution. Then the ph of the solution was adjusted to 6.6±0.2 by adding 10% NaOH solution. This medium was then sterilized by autoclaving for 15 minutes at 121° C under a pressure of 15 lb/inch2 . Finally the prepared medium was transferred on to a water bath to reduce the temperature to 40-50°C. For testing the sensitivity of fungus, this medium was used. The composition and the method of preparation of this medium were as follows:

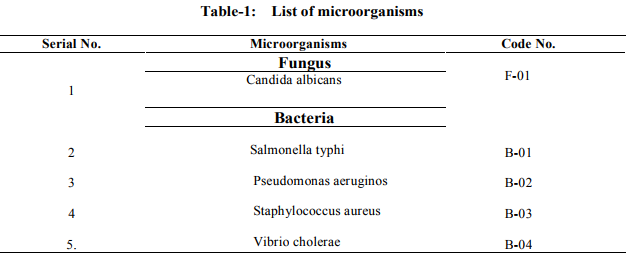
This medium was prepared by dissolving each of the required an amounts of constituents in distilled water. The solution was boiled for about 15 minutes tilt complete dissolution. The pH was adjusted to 7.4 by adding 10% sodium hydroxide (NaOH) solution. The medium was then sterilized by autoclaving 'for 15 minutes at 121 °C under a pressure of 15 Ibflnch2 . Finally the prepared medium was transferred on water bath to reduce the temperature to 45-50%. Test organisms: The bacterial and fungal strains which were used in the sensitivity test are listed in the following table-1. The microorganisms were collected from International Centre for Diarrheal Diseases Research, Bangladesh (ICDDRB), Mohakhali, Dhaka and Drug international pharmaceutical Ltd. Dhaka.
Preparation of sample solution:
1 g each of the dried decolorized extracts (ether, ethyl acetate and alcohol extracts) was dissolved in 5 ml of distilled water to prepare stock solution. As a solubilizing agent, few drops of 1% Tween-80 were used. So the concentration of the solutions was 200 mg/ml. From these solutions, 200 µl i.e. 2 micropipette (1 micropipette= 0.1 ml) of each sample were, taken and applied into each hole or cup on the agar plate. To make a comparative study, ampicilin and gentamicin was taken as a standard as antibiotic and nystatin as antifungal, where the concentration was 200mg/ml.
Inoculation:
Specific organisms were inoculated into 30 ml of previously sterilized nutrient agar media with the help of a sterilized inoculating loop. The inoculated medium was mixed thoroughly and immediately transferred to the sterile Petri-dish in an aseptic condition. The Petri-dish was rotated several times, first clockwise then anticlockwise. A homogenous distribution of the test organism was prepared. After the completion of preparation of medium, it was stored in an incubator for about 24 hours to allow the proper growth of microbes. After proper incubation the Petridis was harvested by sterile saline TS to prepare bacterial suspension.
Preparation of the cups:
The temperature of sterilized antibiotic medium was reduced to 45- 50°C and 100 ml of it was transferred in a bottle. A small volume of bacterial suspension (1-2 ml) was added to the bottle containing l00 ml antibiotic medium.. After proper mixing, a suitable volume (25-30 ml) of this medium was added to a sterilized plate to a layer of uniform thickness (2-3mm.). After solidification of the seeded antibiotic medium, small holes or cups were cut in it with the help of a specific borer. Four types of cups were prepared in each plate and they were-Alcohol extract cup, Alcohol cup, Standard cup (ampicilin/nystatin cup), Standard cup (gentamicin cup). The cups were sufficiently far from each other to prevent overlapping of the zones of inhibition. Prepared sample solutions were applied to the corresponding cups or holes with the help of a micropipette. The plates were then allowed to stand to diffuse the sample solution font the antibiotic medium at room temperature for an hour so that the diffusion of the sample solutions was completed. The plates were then incubated at 37°C for overnight.
RESULT AND DISCUSSION
Microbiological evolution:
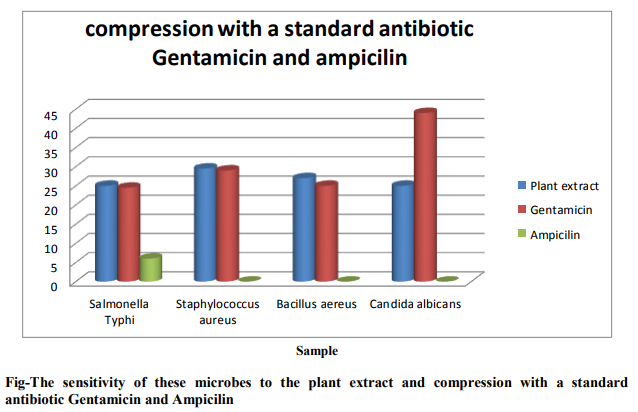
Antimicrobial Activities of the decolorized extracts of Bacopa monnieri (L.) Penn. The in vitro antimicrobial activities of the root extract were tested against 3 pathogenic bacteria and 1 fungus which are responsible for various infection diseases. The sample organisms are indicate by code number as Salmonella typhi (B-1), Staphylococcus aureus (B2), Bacillus aereus (B-3) and Candida albicans (F1). Here, B=Bacteria and F= Fungus Concentration of plant extract =200, Concentration of Gentamicin = 200, Concentration of Ampicilin =200. The Sensitivity of these microbes to the plant extract and compression with a standard antibiotic Gentamicin and Ampicilin shown in flowing bar graph and figure .
Clinical Investigation:
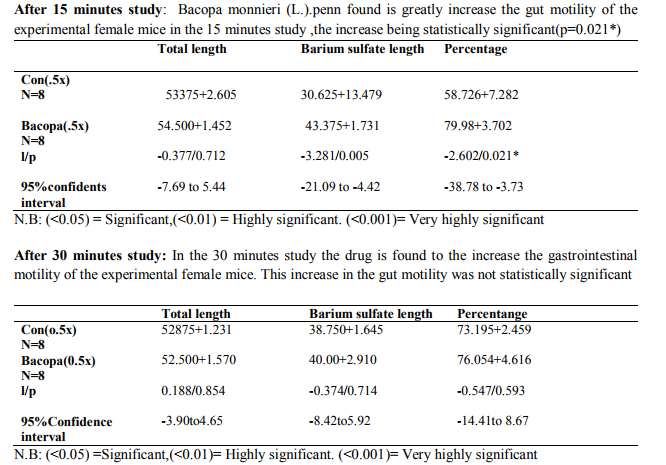
The test was carried out to find out the effect of formulation Bacopa monnieri (L.). Penn extracts to the motility of the gastrointestinal tract. The statistical finding and the Tabular presentation is the effect of the Bacopa monnieri (L.). penn on gastrointestinal motility utilizing female mice. The research outcome can be summarized as follows Percentage traversed after 15 min=GI-motility greatly increased. Percentage traversed after 30 min= GI-motility slightly increased
References:
1. Champion, R.H, et al (1992), A Text book of Dermatology 5th edition, Vol-3, P. 963, Blackwel Scientific Publication, Oxford.
2. Faresworth, (1966), Journal of Pharmaceutical Science, 55 (3): 225.
3. Hill, A.F. (1972), Text book of Economic Botany, TMH edition, P. 243, Tata Publishing Company Ltd. New Delhi.
4. Graves, G. (1990), Text book of Medicinal Plants, 2nd edition, P. 6, Bracken book Publisher,
5. Grover, G.S. and Rao, T.J. (1980), Analysis of Bacopa monnieri (L.) seeds Journal of Industrial Chemistry, Calcutta, 52(5): 176- 178
6. Irvine, S. (1995), In. Pharm, ADIS International Ltd., p. 3-4.Journal of Pharmaceutical Sciences, 55(3): 226, March, 1966.
7. Kenneth, A. C. (1982), A Text book of Pharmaceutical Analysis, 3rd edition, p. 356-357, A Wiley- Inter science Publication, New York.
8. Kiritikar, K.R. & Basu B.D. (1984), Text book of Indian Medicinal Plants-2, 1st edition p. 1568, Langle Medical Publication
9. Kcchhlar.S.L. (1981), A Text book of Economic Botany in the Tropics, 1st edition, P.397-399, published by Me Millan India, Ltd.
10. Sofora, A. (1982), Medicinal Plants and Traditional Medicine in Africa, P 6-8, published by John Willy and Sons Ltd. New York.
11. Sri vastava, O.P. (1984), The Use of Pharmacological Technique for Evaluation of Natural Products, edited by Dewan, B.N. and Srimal, R.C., P. 75.
12. Stahl, E. (1969), Thin Layer Chromatography: A Laboratory Hand Book, 2nd edition, Springer Verlag, New York.
13. Tayler, V.E, Brady, L.R. and Robbers, J.E. (1985), A Text book of Pharmacognosy, 9th edition, p. 159,
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License