IJCRR - 4(20), October, 2012
Pages: 65-72
Date of Publication: 20-Oct-2012
Print Article
Download XML Download PDF
A STUDY ON BIOELECTRICITY GENERATION FROM THE SEA WATER USING MICROBIAL FUEL CELL
Author: Shiv Kumar, Harsh Dev Kumar, Gireesh Babu K
Category: General Sciences
Abstract:Microbial Fuel Cells (MFCs) use bacteria as biocatalyst to convert biodegradable substrates into electricity. The normal sea water generated maximum Open Circuit Voltage (OCV) of 4.5mV while sterile sea water generated only 0.9mV OCV in the H-shaped MFC. On optimization of MFC setup, 100% sea water and a salt bridge (5cm × 2cm) containing a mixture of 10% sodium chloride and 5% agar, and electrodes of 32.20cm2 surface area was found ideal. Furthermore, two electrogenic bacteria were isolated from the sea water and individually studied for their electrogenicity. The isolate SWA1 was selected as electrogenic bacteria, as it generated 754.7mV OCV at 37ºC, pH 7.0 with LB medium as anolyte against vinegar as catholyte after 24h inoculation in MFC. Based on phenotypic characteristics and 16S rDNA sequencing, isolate SWA1 was identified as Pseudoalteromonas sp.
Keywords: Bioelectricity, Electrogenic bacteria, Microbial fuel cell, Open circuit voltage, Sea water
Full Text:
INTRODUCTION
World energy demand is expected to raise from 421quadrillion British Thermal Units (BTUs) in 2003 to 563 quadrillion BTUs in 2015 to 722 quadrillion BTUs in 2030 [1]. Energy production from renewable feed stocks holds great potential to meet these needs in a sustainable and environmentally sound manner and to reduce dependence on fossil fuels. Recently, microbial fuel cells (MFCs) have drawn increasing worldwide attention in directly generating electricity from organic matters [2, 3]. Microbial fuel cells (MFCs) are Bio-Electrochemical Systems (BESs) [4], which utilize bacteria to oxidize organic matter and transfer electrons to the anode, where they flow to the cathode and react with protons and oxygen to form water [5]. Bacteria which are useful in microbial fuel cell operation have the ability to transfer electrons to an electrode (anode), as a terminal electron acceptor are classified as Electrochemically Active Bacteria (EAB) or Electrogenic bacteria [6]. Conventional methods of isolating exoelectrogenic microorganisms are based primarily on identifying microorganisms that can respired using soluble or insoluble metal oxides in agar plates [7, 8]. However, not all dissimilatory metal oxide reducing bacteria are capable of producing electricity in an MFC, and not all bacteria that produce current in an MFC can grow using metal oxides [9,10]. Therefore, these methods may miss important electrochemically active strains of microorganisms. Until date, several reports have been documented on the electricity generation from the different natural waste matter like land fill, sea sediments and waste water; however a very less reports are available where normal sea water samples containing bacteria while organic and inorganic compounds in it used as fuel to monitor its potential for the electricity generation using two chambers MFC. The present study done focus on the determination of electric potential of normal sea water to generate the electricity in fabricated two chamber MFC followed by screening and identification of potent electrogenic bacteria present in it.
METHODS AND MATERIALS
Collection of samples
The sea water sample was collected in sterile containers from the Kanyakumari,Tamil Nadu, India whereas a liter of sewage wastewater from Meerut, Uttar Pradesh, India was also collected, carry and stored at 4ºC until employed in the experiment. Initially, the studies were carried with sea sediment samples as a sole anolyte against vinegar, to note the bioelectrical contribution of the microbial flora present in it.
MFC fabrication and operation
The H-shaped MFCs were fabricated with two polycarbonate bottles (500 mL) as chambers and a PVC pipe (5cm × 1cm) for preparing a salt bridge. The slat bridges were prepared by filling boiled sodium chloride (10%) solution containing 5% agar. The salt bridges were fixed to the bottles with the aid of epoxy adhesive (M-Seal, Pidilite Industries Ltd, Maharashtra, India). The sea water was used as anolyte without any pretreatment and vinegar was used as a catholyte in the MFC setups. The two graphite pencils (0.25cm × 10cm, Apollo pencil manufacturers, Mumbai, India) with surface area of 16.10cm2 were used as electrodes. To aid initial wetting, the electrodes were boiled in deionized water and soaked overnight in 1M HCl solution followed by thorough rinsing with deionized water [11]. The electrodes were inserted into respective chambers while circuit connections were set with the copper wires fixed into the drilled holes of the electrodes and sealed with epoxy resin to avoid corrosion of copper wire [12]. The fabricated MFCs were sterilized with Ethanol (70% v/v) and irradiated with UV for 15 min followed by electrolytes addition up to the brim of the respective chambers. After 24h, OCV from the MFCs was recorded at room temperature without any external resistance until 40min with an interval of 2min using the multidigital meter (UNI-DT830D, Uni-Trend Group Ltd., Kowloon, Hong Kong). Optimization of MFC operation Since, it is necessary that MFCs should be optimized in terms of reactor configuration and physiochemical parameters, MFCs were operated using different concentrations of sea water (100, 75, 50, and 25%), 10% concentration of different salts; sodium chloride(NaCl), potassium chloride(KCl), ammonium nitrate(NH4NO3), ammonium chloride(NHCl4) and mixed salts (2.5% each salt), different concentrations of optimized salt (5, 10,15 and 20 %), different lengths of salt bridge (1.5, 3 and 5cm), various radius of salt bridge (1cm and 2cm),different surface area of anode (16.1cm2 and 32.2cm2 ), exogenous mediators (phenol red and neutral red) while potential difference was monitored in terms of OCV. During the optimization experiment, the optimized parameter was employed in the optimization study of another parameter.
Sea water verses sewage water
The optimally fabricated MFC setup was monitored for the OCV generation with the sea sediments as anolyte against 500mL of sewage water as catholyte in the respective chambers of the MFC as described earlier section. Isolation of bacteria from sea sediment The sea water sample was serially diluted with saline water (0.85% w/v NaCl) and the 10-5 dilution of the sample (0.1mL) was then spread on the LB agar plate (Tryptone 10.0g, NaCl 10.0g, Agar 20.0g and Yeast extract 5.0g in 1000mL distilled water) further incubated for 24h at 35°C. Morphologically distinct bacterial colonies were purified and further studied for their Gram staining properties [13]. Physiochemical growth optimization of bacteria The LB broth prepared were inoculated with the 24 h old sea water isolates culture (1 %, v/v) separately were studied for the bacterial growth at different pH (4, 5, 6, 7, 8, and 9) and temperature (27, 37, 47 and 57ºC) by measuring absorption at 660 nm, against the sterile LB broth as blank [14]. Electricity generation and identification of potent electrogenic bacteria Using optimized growth and MFC parameters, the isolates were studied for their electrogenicity with 500mL LB broth culture (24h old) as anolyte against 500mL vinegar as catholyte in terms of OCV. The potential isolate screen out was then outsourced to Bioserve Biotechnologies Pvt. Ltd, Hyderabad, India, for molecular identification by 16S rDNA technique. The 16S rDNA sequence obtained was initially analyzed at NCBI server (http://www.ncbi.nlm.nih.gov/) using BLAST (Blastn) tool and corresponding sequences downloaded were further used for phylogenetic analyses using MEGA version 4 [15].
RESULTS
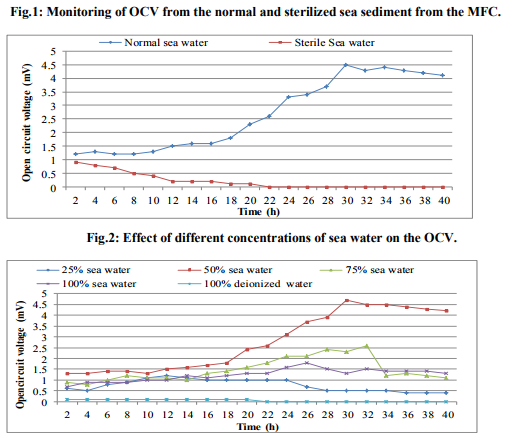
Analysis of electricity from sea water
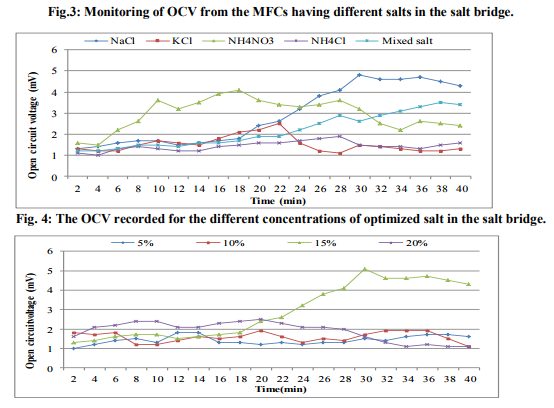
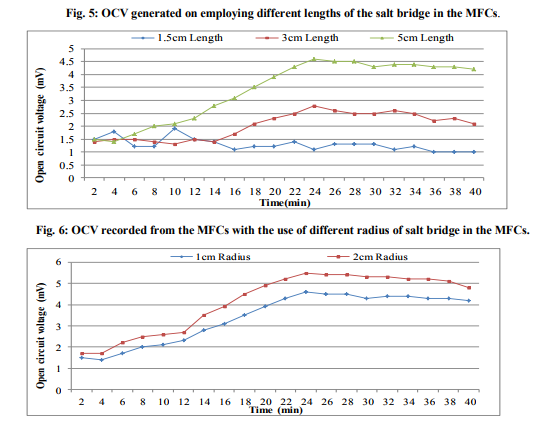
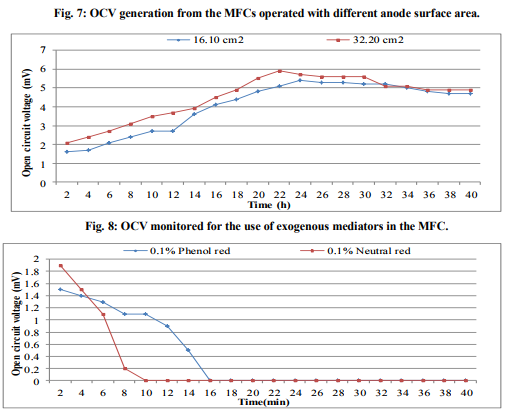
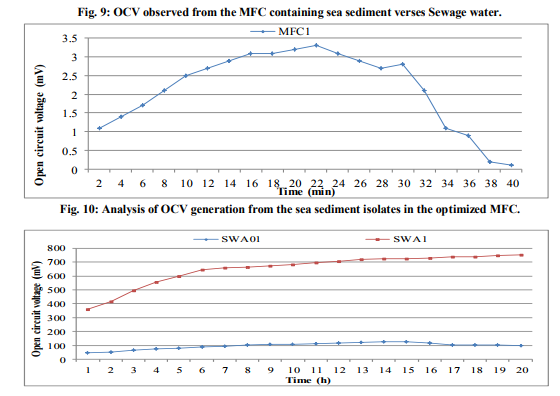
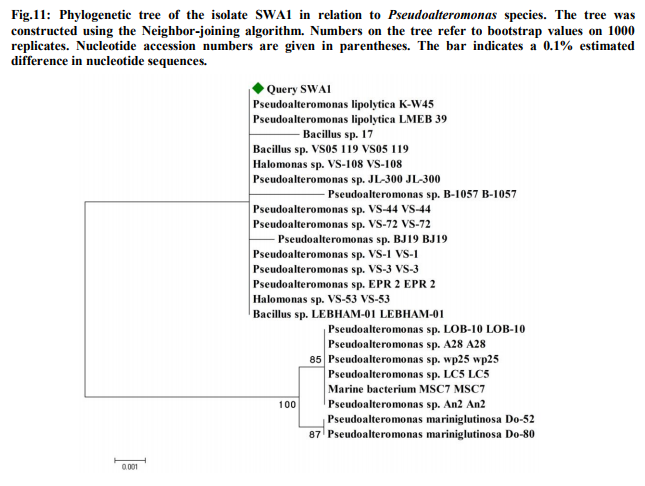
In order to check the presence of electrogenic bacteria in the sea water samples, electricity generation monitored from the normal sea water samples and sterilized sea water was observed against vinegar (Fig.1). The maximum OCV of 4.5mV was recorded at 16min from the normal sea water while highest OCV profile of 0.9mV at 2min was monitored from the sterilized sea water. This deduced that the normal sea water samples possessed the electrogenic bacteria which contributed into the electricity production. Analysis of MFC parameters Concentrations of sea water The sea water sample was diluted with deionized water to various concentrations served as anolyte in the MFCs where OCV was monitored (Fig.2). It was observed that maximum OCV of 4.7mV at 18min was recorded from the MFC containing 100% sea water. Effect of different salts and concentrations of ideal salt Among the different salts studied in the salt bridge having 10% concentration, the maximum OCV of 4.8mV was recorded by the salt bridge containing NaCl (Fig.3) at 18min with continuous and steady elevation in the electric potential over time in comparison to other salts. Hence, the NaCl was opted as the suitable salt which was further analyzed for its effective concentration for better OCV. The concentration of NaCl was further varied and observations revealed that 10% could yield a maximum OCV of 4.9mV at 19min with consistent increment over time (Fig.4). Dimensions of salt bridge (Length and radius) Based on optimized NaCl concentration, various lengths of the salt bridge were monitored for the MFC. Fig.5 illustrates highest OCV generation of 4.6mV from the 5cm long salt bridge with a uniform increment over time. The salt bridges having 5cm length were varied in radius and used in the MFC setups. The OCV profile (Fig.6) reveals that salt bride 2cm radius could generate more OCV of 5.5mV at 17 min in comparison to that of 1cm radius salt bridge. Anode surface area The effect of anodic surface area of 16.10cm2 and 32.2cm2 were used to check its effect on the OCV from MFC setup. The data obtained revealed that maximum OCV of 5.9mV was recorded with 32.2cm2 anodic surface area at 16min (Fig.7). The results concluded that anode with 32.2cm2 surface area was ideal for the MFC configuration. Analysis of exogenous mediator The electricity generations in terms of OCV were recorded with phenol red and neutral red amended as electron shuttles in the anodic chamber containing 100% sea water as anolyte. The maximum OCV of 1.5mV and 1.9mV were monitored for the phenol red and neutral red at 2min, respectively followed by sudden decrease in the potential (Fig.8). Analysis of electricity generation from sea water against sewage water The fabricated MFC setup was operated with all the optimized parameters studied and production of electricity was recorded after 24h of inoculation in terms of OCV from MFC with 100 % sea water as anolyte against 500mL untreated sewage water as catholyte. It was observed that MFC with sewage water as catholyte could produce (Fig.9) maximum OCV of 3.3mV at 13min but fluctuations in the reading was recorded. Study on sea water bacterial isolates The two morphologically distinct bacterial colonies isolated were named as SWA01 and SWA1. Gram staining study revealed that the sea water isolate SWA01 was gram-negative cocci and SWA1 was gram-negative rods in nature. The pH 7 and temperature 37ºC were recorded as the optimized growth requirements for both bacterial isolate. Analysis of electricity generation from the sea sediment bacterial isolates The isolates were independently studied for their potential to produce bioelectricity in the MFCs. Fig.10 represents the OCV generation from the 24h old bacterial isolates cultures recorded for 40 min at the 2 min intervals, inoculated as anolyte in the anodic chambers. The MFC containing isolate SWA1 showed the maximum OCV of 754.7mV at 40min, with significant steady increase over time in comparison to SWAO1 in the MFCs. Identification of isolates SWA1 The bacterial colonies of the isolate SWA1 observed were smooth, circular, light red in color, 1mm in diameter. Furthermore, sequence analysis of the 16S rDNA showed 100% similarity with the Pseudoalteromonas lipolytica strain K-W45 (JQ79909). Based on the phenotypic characteristics and phylogenetic analysis using 16S rDNA, isolate SWA1 was identified as Pseudoalteromonas sp. The Fig.11 shows the phylogenetic relationship between the isolate SWA1 with the Pseudoalteromonas sp. The 16S rDNA sequences for the isolate SWA1 have been deposited at Gene Bank in NCBI with accession number JX105433.
DISCUSSION
The present study concluded that the sea water harbor electrogenic bacteria which lead to the production of bioelectricity in the fabricated MFCs. The 100% sea water produced more OCV must be because of less number of bacteria in the diluted concentration of sea water. This analysis of different salts for the Salt bridge recommended the use of 10% NaCl concentration as optimized salt in the salt bridge to facilitate easy ion flux. The sodium chloride in the salt bridge yielded good OCV might be because of its good electrolytic property [16]. While 5cm length of salt bridge proved better with 2cm diameter might be because of less density of agar in longer length and easy flow ions through wider diameter. In the fabricated MFC, results concluded that use of anode with 32.2cm2 surface area showed more OVC, must be because of more space for the bacteria to liberate the electron. Also, both the exogenous mediators used showed decrease in the OCV. It was reported that most of the exogenous mediators are toxic for the microbes [2]. Electricity generation from the sea water and sewage could not produce good yield might be effect of ions present in both the electrolytes. The bioelectricity generation from the sea water bacterial isolates results indicated that isolate SWA1 contributed more effectively into overall voltage generation from the normal sea water where other strain also generate the potential but fluctuations were observed in the reading obtained during MFC operation. The OCV of 754.7mV was generated by the isolate Pseudoalteromonas sp. SWA1 was nearer to that of maximum OCV of 800mV [16] reported until now. This implies that the bacterial isolate SWA1 might have electrogenic properties like C-type cytochromes [17] or conductive nano-wires [18] on its cell membrane to generate electric potential. Also the optimum pH noted for the SWA1 was in accordance with the report in which highest bioelectricity generation observed thus far at pH 7.0 [19].
CONCLUSION
The present study revealed that the potential electrogenic bacteria can be easily procured from the natural sources like sea water by simple technique of serial dilution and spread plate for operating the MFCs other than sediment or benthic MFCs. The study recommends the further tuning of the technology for the better usage of the potential electrogenic bacterial isolate from the sea water towards the commercial utilization to generation of alternate energy to sustain the demand of future.
References:
1. Khanal SK. 2008, Anaerobic Biotechnology for Bioenergy production: Principles and applications, John Wiley and Sons Inc. ISBN978- 0-813-82346-1 [Chapter 10, Microbial Fuel Cell: Novel Anaerobic Biotechnology for energy generation from waste water, Hong Liu, 221-246].
2. Rabaey K, Lissens G, Siciliano S D, Verstraete W. A microbial fuel cell capable of converting glucose to electricity at high rate and efficiency. Biotechnol Lett 2003; 25:1531-1535.
3. Liu H, Logan BE. Electricity generation using an air cathode single chambers microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 2004; 38:4040-4046.
4. Rabaey K, Rodr?´guez J, Blackall LL, Keller J, Gross P, Batstone D, et al. Microbial ecology meets electrochemistry: electricity-driven and driving communities. ISME J 2007; 1:9-18.
5. Logan BE, Hamelers B, Rozendal R, Schroder U, Keller J, Freguia S, et al. Microbial fuel cells: methodology and technology. Environ Sci Technol 2006; 40: 5181-5192.
6. Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W. Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 2004; 70:5373-538.
7. Logan BE. 2008. Microbial fuel cells. John Wiley and Sons, Inc., New York, NY.
8. Lovley D R. Bug juice: harvesting electricity with microorganisms. Nat Rev Microbiol 2006; 4:497-508.
9. Richter H, Lanthier M, Nevin KP, Lovley DR. Lack of electricity production by Pelobacter carbinolicus indicates that the capacity for Fe(III) oxide reduction does not necessarily confer electron transfer ability to fuel cell anodes. Appl Environ Microbiol 2007; 73:5347-5353.
10. Bretschger O, Obraztsova A, Sturm CA, Chang IS, Gorby YA, Reed SB, et al. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl Environ Microbiol 2007; 73:7003-7012.
11. Allen RM, Bennetto HP. Microbial Fuel Cells: electricity production from carbohydrates. Appl Biochem Biotech 1993; 39(40):27-40.
12. Zou YJ, Sun LX, XU Fen, Yang Li-Ni. E.coli microbial fuel cell using new methylene blue as electron mediator. Chemical Journal of Chinese Universities 2007; 28(3):510-513.
13. Smibert RM, Krieg NR. Phenotypic characterization in methods for general and molecular bacteriology. American Society for Microbiology 1994; 611-651.
14. Safia M, Xama K, Madamwar D. Isolation, characterization and decolorization of textile dyes by a mixed bacterial consortium JW-2. Dyes and Pigments 2007; 74:723-729.
15. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 2007; 24:1596-1599.
16. Liu H, Cheng S, Logan BE. Production of electricity from acetate or butyrate using a single-chambers microbial fuel cell. Environ Sci Technol 2005; 39:658-662.
17. Kim HJ, Park HS, Hyun MS, Chang IS, Kim M, Kim BH. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb. Technol 2002; 30 (2):145-152.
18. Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci USA 2006; 103:11358-11363.
19. Gil GC, Chang IS, Kim BH, Kim M, Jang JK, Park HS, et al. Operational parameters affecting the performance of a mediator-less microbial fuel cell. Biosensors and Bioelectronics 2003; 18, 327-334.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License