IJCRR - 4(23), December, 2012
Pages: 88-94
Date of Publication: 15-Dec-2012
Print Article
Download XML Download PDF
EFFECTS OF ANTI-PROGESTERONE ON PROGESTERONE AND OESTROGEN RECEPTORS PRESENT IN STROMAL CELLS OF RAT UTERUS
Author: Khadija Qamar, Iram Iqbal, Umbreen Noor, Ifra Saeed, Humaira Arshad, Farheen Masood
Category: Healthcare
Abstract:Objective: To investigates the effect of mifepristone (anti-progesterone) on stromal cells of the uterus of rats. Study Design: Laboratory based randomized controlled trials Method: Sixty adult female rats were divided randomly into two groups, comprising of 30 animals in each group. In-group A 1ml of normal saline was given orally daily for three months while in group B mifepristone was given orally in a dose of 1mg/kg body weight daily for three months. All the animals were sacrificed next day after the last oral dose. 2ml blood was taken directly from the heart for measurement of progesterone levels. Sections were stained with hematoxylin and eosin for light microscopic study. Immunohistochemical staining procedure was done for demonstration of progesterone receptors Results: The stromal cells were flattened and irregular in outline present around the glands. Some appeared fusiform or spindle shaped. In the experimental group the stromal cells were tightly packed. There was an increase in the number of infiltration of granulocytes and eosinophils in the stroma. Progesterone antagonist application lowered the plasma concentration of progesterone. The number of progesterone receptors in all uterine compartments of the experimental group were decreased and found statistically significant. Conclusion: In conclusion, mifepristone affects stromal, glandular and epithelial morphology in the rat uterus.
Keywords: Mifepristone, receptors, oestrogen, progesterone, stromal cells
Full Text:
INTRODUCTION
Oestrogen effects predominate during the follicular phase of the estrous cycle, whereas progesterone dominates during the proliferative phase in non-pregnant uterus1 . Oestrogen & progesterone have specific intracellular receptors members of the nuclear receptor super family of transcription factors2 . Estraneprogestins are the precursors of Mifepristone, {17(,hydroxyl 11-(-(4- dimethylaminophenyl)-17(-(prop-1-ynyl)-estra-4, 9-dien-3-one,}3 . Mifepristone binds strongly to the progesterone and glucocorticoid receptors, and to a lesser extent to the androgen receptor, thus mediating its effects at the receptor level4 . This study was conducted to investigate the effect of mifepristone (anti-progesterone) on stromal cells of the uterus of rat.
MATERIAL AND METHODS
These laboratory based randomized controlled trials were conducted at the department of Anatomy, Army Medical College Rawalpindi from Jan 2007 to March 2007. Institutional Ethical Committee of Army Medical College Rawalpindi approved all the procedures. Sixty healthy adult female Sprague Dawley rats weighing 200-300g were procured from the National Institute of Health Sciences Islamabad. The animals were randomly divided into two groups having 30 rats in each.
Group A (Control)
Thirty female rats were given 1ml of normal saline orally daily for three months.
Group B (Experimental)
Thirty female rats were given the drug (Mifepristone) orally in a dose of 1mg/kg body weight daily for three months. All the animals were sacrificed next day after the last oral dose. 2ml blood was taken directly from the heart for measurement of progesterone level. About ½cm piece of tissue was taken from the middle of the right uterine horn. Approximately five microns thick sections were cut and stained with hematoxylin and eosin for light microscopic study. Immunohistochemical staining procedure was done for demonstration of progesterone receptors.
Microscopic observations
Qualitative Parameters
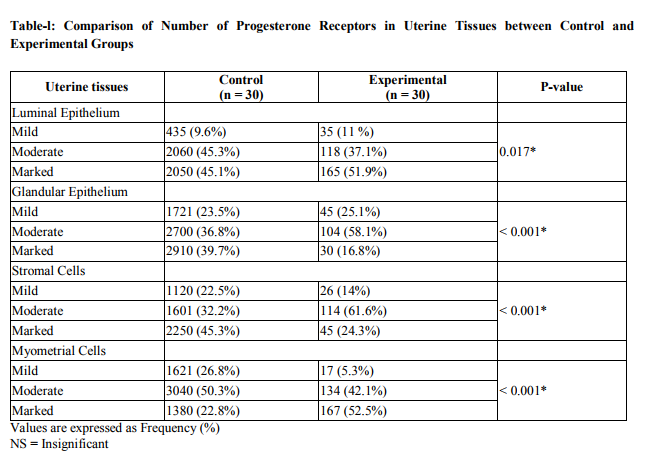
Morphology of the distribution of stroma was noted.
Statistical analysis
Data had been analyzed using SPSS version 15. Descriptive statistics were used to describe the data. Quantitative variables were compared through independent samples’ t-test while qualitative variables were compared through chisquare test between cases and controls. P-value < 0.05 was considered as significant.
RESULT
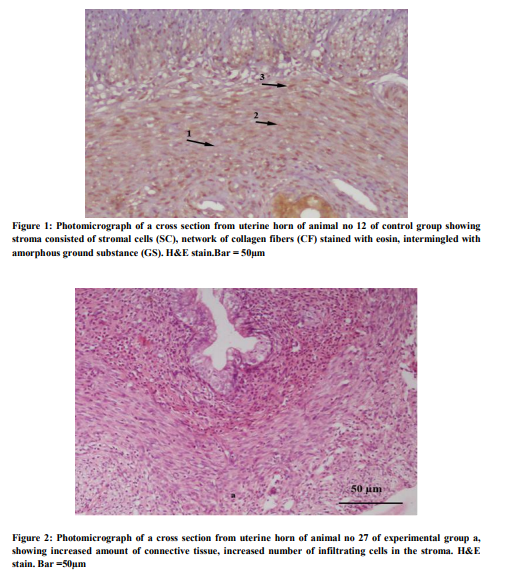
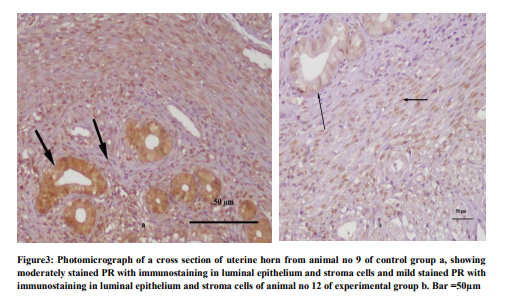
Total 60 animals were included in the study, 30 in each group. In the control group the tubular sections showed three distinct layers (inner, middle and outer) of endometrium. The luminal side of inner layer was lined by single regular row of cylindrical cells. The stroma consisted of stromal cells, network of collagen fibers stained with eosin, intermingled with amorphous ground substance, and several blood vessels (Fig 1). The stromal cells were flattened and irregular in outline present around the glands. Some appeared fusiform or spindle shaped. They had ovoid nuclei with acidophilic cytoplasm. The eosinophils were recognized by their specific pink stained granules. The lymphocytes with deep staining indented nucleus were also present. While in experimental group at low magnification, inner layer was folded giving it an overall ruffled appearance as a result lumen was much reduced as compared to normal group. Epithelium was low columnar. The superficial part of inner layer consisted of few glands and abundant stroma and a deeper layer had many glands and relatively less stroma. The stromal cells were tightly packed, having basophilic cytoplasm. The infiltration of the stroma with the eosinophils was observed. (Fig.2). Glands varied in size, some of them were dilated and cystic. The mean progesterone level in experimental group was 2.8 + 0.09 ng/ml, which was lower than that of the control group i.e. 5.5 ± 0.8. The difference was found statistically significant when compared with control group (p =0.001). The mild, moderate and marked progesterone receptors were counted in the luminal epithelial cells, glandular epithelial cells, stromal cells and myometrial cells. The number of progesterone receptors in all the compartments of the uterus was reduced in the experimental group as compared with the control group and found statistically significant (Fig.3) (Table-l).
DISCUSSION
The direct effect of the hormone is usually responsible for all the changes seen in the luminal epithelium in response to antiprogesterone5 .
The changes in the uterine wall are likely due to changes in the epithelial – stromal cell interactions and growth factor actions that are required for uterine development6 . Mifepristone suppresses endometrial cell proliferation and thus, causes reduction in overall endometrial thickness7 . Cyclical endometrial proliferation, differentiation and secretion during the human menstrual cycle are strictly controlled by oestrogen and progesterone. A balance is required between oestrogen and progesterone production to ensure normal endometrial growth and proliferation8 . The absence of progesterone removes the ‘progesterone brake’ leading to persistent estrogenicity and constant endometrial proliferation. Although the ratio of stroma to glands remains normal. The endometrium can become disordered and vascular abnormalities such as dilated capillaries may become apparent9 . T he effects of unopposed estrogen may lead to endometrial hyperplasia and possible malignancy, a fact to be kept in mind with long-term use of PR antagonists. In rats receiving long-term PR antagonist treatment estrogenic stimulation of the endometrium has been recorded10 .The nonhuman primate endometrium, however, demonstrates endometrial atrophy and evidence of antiestrogenic. High doses of mifepristone (25mg/d and 50mg/d) cause variable effects, such as atypical cystic changes in eutopic endometrium11 . Serum progesterone levels declined in experimental groups after mifepristone administration. No statistically significant changes in the progesterone levels were observed in the 2-day following the administration of 200mg mifepristone12 . However, with 600mg of mifepristone administered progesterone levels increase on day 1 and then decrease significantly13 . The paradoxical effects (Mifepristone both raises and lowers progesterone levels) have also been explained by the hypothesis, that mifepristone can act either by preventing the progesterone effect or in a way that is similar to that of progesterone, which always stimulates its own secretion by autoregulation. The effects may be variable depending on the duration of pregnancy14. The highest density of stromal cells nuclei was observed in all the uteri of the experimental group. The stromal cells were tightly packed with scanty basophilic cytoplasm. The infiltration of the stroma with the eosinophils was observed. The increased level of leukocytes may not be a direct effect of antiprogestin. Mifepristone treatment may up-regulate potential chemokines that cause leukocyte traffic and interleukin-8 and an increase in MCP-1 and influence leukocyte influx in human decidua15 . Dependent stromal compaction could be identified as one of the key morphological features of the antiprogestrogenic action of mifepristone. It is well established that the proliferation of endometrial stroma is progesterone - dependent in rat. Considering that both the oestrogen and progesterone receptor proteins are oestrogen-dependent, in the present study any change in the concentration or localization of endometrial ER and PR was determined to evaluate whether the antiprogestininduced anti-oestrogenic effects are reflected in these receptors16. The most obvious change in steroid receptor distribution in the uterus was the reduction in staining intensity PR in the glandular epithelium and stromal cells. An increase in the number of endometrial ER and PR has been observed after chronic administration of antiprogestin17. Progesterone receptor concentration could be expected to be highly associated with successful implantation since many of the relevant local factors such as cytokines and growth factors are progesteroneregulated. Endometrial receptivity is strongly related to the down regulation of the progesterone receptor.
CONCLUSION
In the experimental group the stromal cells were tightly packed. There was an increase in the number of infiltration of granulocytes and eosinophils in the stroma. Progesterone antagonist application lowered the plasma concentration of progesterone. The number of progesterone receptors in all uterine compartments of the experimental group were decreased and found statistically significant. In conclusion, mifepristone affects stromal, glandular and epithelial morphology in the rat uterus.
ACKNOWLEDGEMENTS
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Connelly OM. Female Steroid Hormone Action. Endocrinology 2001; 142(6): 2194- 99.
2. Benakanakere C, Besch-Williford M, Ellersieck R, Hyder SM. Regression of progestin-accelerated7, 12- dimethylbenz [a] anthracene-induced mammary tumors in Sprague-Dawley rats by p53 reactivation and induction of massive apoptosis, a pilot study. Endocr Relat Cancer; March 1, 2009; 16(1): 85-98.
3. Sukjum LS. Studies of oestrogen and progesterone receptors in the sow uterus with special emphasis on the oestrous cycle and early pregnancy [PhD dissertation], Vpp sala. Swedish university of Agricultural Sciences 2005; p 12-17.
4. Antoniou G, Kalogirou D, Karakitsos P. Transdermal estrogen with a levonorgestrelreleasing intrauterine device for climacteric complaints versus estradiol releasing vaginal ring with a vaginal progesterone suppository, Clinical and endometrial responses. Maturities 1997; 26: 103-11.
5. De Vivo I, Hankinson SE, Colditz GA, Hunter DJ. A functional polymorphism in the progesterone receptor gene is associated with an increase in breast cancer risk. Cancer Res 2003; 63: 5236–8.
6. Gopalkrishnan K, Katkam RR, Sachdeva G, KholKute SD, Padwal V, Puri CP. Effects of an Antiprogestin Onapristone on the Endometrium of Bonnet Monkeys, Morphometric and Ultra Structural Studies. Biol Reprod 1959; 68(6): 779-87.
7. Brenner RM, Slayden OD. Estrogen action in the endometrium and oviduct of rhesus monkeys during RU 486 treatment. In: Beier HM, Spitz IM, and editors. Progesterone antagonists in reproductive medicine and oncology. Cary, NC. Oxford University Press 1994; p.82-97.
8. Jeffrey R, Goldberg, MD, Marcus G, Plescia MD, MPH, Geraldine D Anastasio. Mifepristone (RU 486) Current Knowledge and Future Prospects. Arch Fam Med 1998; 7: 219-222.
9. Van Look PFA, Ven herizen H. Postovulatory methods of fertility regulation: the emergence of antiprogestens. In Van Look PFA, Perez – Polacies G, editors. Contraceptive research and development. New Delhi, Oxford University Press 1994; P. 151-201.
10. Baird DT, Glasier AF. Science, medicine and the future contraception. Br Med J 1999; 319: 969-72.
11. Hargrove JT, Maxson WS, Wentz AC, Burnett LS. Menopausal hormone replacement therapy with continuous daily oral micronized estradiol and progesterone. Obstet Gynecol 1989; 73: 606-12.
12. O.D. Slayden, M.B. Zelinski, K. Chwalisz, H. Hess-Stumpp, R.M. Brenner Chronic progesterone antagonist–estradiol therapy suppresses breakthrough bleeding and endometrial proliferation in a menopausal macaque model .Hum. Reprod. 2006; 12: 3081-3090.
13. S. Schäfer-Somi, O.A. Aksoy, H.B. Beceriklisoy, A. Einspanier, H.O. Hoppen , S. Aslan. Repeated induction of abortion in bitches and the effect on plasma concentrations of relaxin, progesterone and estradiol-17β. 2007; 68: 889-895.
14. Murphy AA, Castellano PZ: RU 486: pharmacology and potential use in the treatment of endometriosis and leiomyomata uteri. Curr Opin obstet Gynecol 1994; 6(3): 269-78.
15. Astrid Petersen, et al: The antiprogesterone Org 31710 inhibits human blastocystendometrial interactions in vitro. Fertility and Sterility 2005; 83: 1255-1263.
16. Shwad PC, Katkam RR, Hinduja IN, Chwalisz K, Elger W, Puri CP. Treatment with a progesterone antagonist ZR>98.299 delays endometrial development without blocking ovulation in bonnet monkeys. Contraception 1993; 48: 57-70.
17. Itruk-Ware R. Approval of Mifepristone (RU486) in Europe. Zentraibl Gynakol 2000; 122(5): 241-7
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License