IJCRR - 5(5), March, 2013
Pages: 101-104
Date of Publication: 22-Mar-2013
Print Article
Download XML Download PDF
A STUDY OF MEASLES VACCINE STORAGE CONDITIONS AT BELLARY DISTRICT
Author: Aravind Karinagannanavar, Bellara Raghavendra, Wahid Khan, Vandana Hiregoudar, T. Gangadhara Goud
Category: Healthcare
Abstract:Background: The vaccine must be stored at refrigerator temperature 2o\?8oC. Reconstituted vaccine should be used immediately. There would be loss of potency and complications such as Toxic shock syndrome if used beyond 6 hours of reconstitution.The weakest chain is Primary health Centres and Subcenters. WHO recommends that nothing should be stored in refrigerator other than vaccines but it hardly happens. Objective: To assess the storage conditions of measles vaccine at all the PHC's of Bellary District. Material and Methods: A Cross sectional study was conducted from May 2010 to April 2011 at areas covered by 53 PHC/PHU of Bellary district. The data was collected by using pretested semi structured questionnaire. Results: All the PHC's/PHU's had maintained temperature 2-80c and had temperature monitoring twice daily for measles vaccine. But only 26 PHC's had maintained temperature book till date. 30 PHC's/PHU's had proper placement of measles vaccine. Conclusion: All the PHC's/PHU's had maintained the vaccine at proper temperature but proper location of the measles was not satisfactory.
Keywords: Measles vaccine, Storage condition
Full Text:
INTRODUCTION
In2002, the World Health Organization estimated that 1.4 million of deaths among children under the age of five were from vaccine-preventable diseases. With 100% immunization, and 100% efficacy of the vaccines, one out of seven deaths among young children could have been prevented.1 Vaccination can contribute substantially to achieving the Millennium Development Goal of reducing the mortality rate among children under five by two thirds between 1990 and 2015. Measles is a leading cause of childhood morbidity and mortality accounting for nearly half the global burden of vaccine preventable deaths. In 2007, there were 197000 measles deaths globally nearly 540 deaths every day or 22 deaths per hour. 2 Measles vaccine must be shipped with refrigerant to maintain a temperature of 10oC or less at all times. Vaccine must be refrigerated immediately on arrival and protected from light at all times. The vaccine must be stored at refrigerator temperature 2o –8 oC, but may be frozen. After reconstitution, measles vaccine must be stored at refrigerator temperature and protected from light. Reconstituted vaccine should be used immediately. If reconstituted vaccine is not used within 6 hours, it must be discarded. There would be loss of potency and complications such as Toxic shock syndrome if used beyond 6 hours of reconstitution.The weakest chain is Primary health Centres and Subcenters. It is because of lack of proper logistics and lack awareness. WHO recommends that nothing should be stored in refrigerator other than vaccines but it hardly happens. In many health facilities many items like drinking water, food, laboratory items etc will be stored. As no study has been done on storage conditions of measles vaccine in Bellary district an effort is made to study the same.
OBJECTIVES
To assess the storage conditions of measles vaccine at all the PHC’s of Bellary District.
MATERIAL AND METHODS
Topography: Bellary district is spread from southwest to northeast and is situated on the eastern side of Karnataka state. In 2011, Bellary had population of 2,532,383 of which male and female were 1,280,402 and 1,251,981 respectively. Total area under Bellary district is of about 8,447 sq. km.3 A Cross sectional study was conducted from May 2010 to April 2011in Bellary district. Bellary district has 7 talukas, in that 47 primary health centers (PHC) and 6 primary health units (PHU), all of which were studied. Data was collected using a pretested semi structured questionnaire. It includes temperature at which vaccine is stored at PHC, Temperature monitor, maintaince of temperature book, storage of unused vaccines, Technical support when there is power cut and location for the vaccine with in the refrigerator. Data was analysed using epi-info software version 3.4.3. Before the start of study Institutional Ehical clearance was obtained.
RESULTS
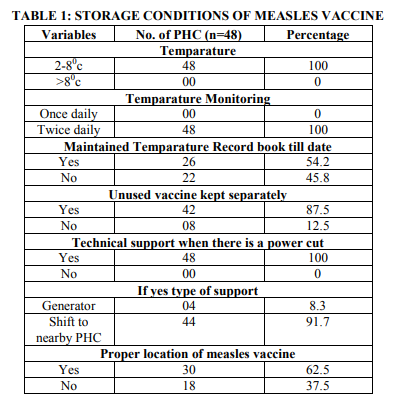
Study included 53 PHC’s/PHU’s, out 53 PHC’s/PHU’s 5 PHC’s/PHU’s were recently upgraded and get vaccines from nearest PHC so only 48 PHC’s/PHU’s were studied. The study shows that all the PHC’s had maintained temperature 2-8 0 c and had temperature monitoring twice daily. But only 26 (54.2%) PHC’s had maintained temperature book till date. 42(87.5%) PHC’s had kept unused vaccines separately. All PHC’s had technical support when there is power cut more than 48 hours among them 4 had generator facility and remaining PHC’s used to shift the vaccine to the nearest PHC. 30 (62.5%) PHC’s had proper location of measles vaccine, in remaining PHC’s T series vaccines and Measles vaccine were kept along with other things like culture media, food and all.
DISCUSSION
Current study shows that all the PHC’s had maintained temperature at 2-8 0 c and had temperature monitoring twice daily. But only 54.2% PHC’s had maintained temperature book till date. 87.5% PHC’s had kept unused vaccines separately. All PHC’s had technical support when there is power cut more than 48 hours among them 4 had generator facility and remaining PHC’s used to shift the vaccine to the nearest PHC. 62.5% PHC’s had proper location of measles vaccine in remaining PHC’s vaccine was with T series along with other things like culture media etc. Similar reports were found by studies conducted across the world to quote few a study conducted by Berhane Y et al., showed that, complete temperature record was observed in more than half of the centres. Vaccine storage in the refrigerator was not proper in three fourth of the centres4 and Grasso M et al., showed that, out of 52 primary vaccination offices inspected, around three fourth centres had a refrigerator for vaccine storage and other faulty procedures, such as the storage of food and laboratory specimens in vaccine refrigerators and the storage of vaccines on refrigerator door shelves.5 This was also found in the present study, so the person incharge and the PHC doctors need to be orient about the importance of vaccine storage according to WHO standards and also not to keep any other items along the vaccines. Pai HH et al. showed that the majority stored articles other than vaccines in their vaccine refrigerators. 25 clinics (39.7%) equipped their refrigerators with UPS (uninterruptable power system). But in our study only 4 PHC;s had this facility and also they discovered inappropriately high temperatures (> 8 degrees C) in 22% of all refrigerators.6 The main problem is lack of orientation to the health personal which was shown by Thakker Y et al., they showed that out of the 40 respondents, only 16 were aware of the appropriate storage conditions for the vaccines; eight had minimum and maximum thermometers but only one of these was monitored daily.7 So there is a need reorient the health personal regarding importance of vaccine potency and the proper storage of the vaccine.
CONCLUSION
All the PHC’s/PHU’s had maintained the vaccine at proper temperature and also monitored the temperature twice daily, but the temperature book was not updated in many PHC’s. And also many PHC’s didn’t have proper location of the measles vaccine and it was kept other things like culture media, food etc. So there is a need for reorientation of the staff regarding proper storage conditions of Measles vaccine.
ACKNOWLEDGEMENT
The authors thank all the staff members of community medicine of VIMS Bellary for there guidance and support. The authors are also grateful to authors/editors/ publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Vaccine-preventable diseases. From Wikipedia, the free encyclopaedia available from www.who.int/immunisation_monitoring/ diseases/en.
2. WHO (2008), Fact sheet no 286, WHO website
3. Bellary District From Wikipedia, the free encyclopaedia available from http://en.wikipedia.org/wiki/Bellary_distr ict. Cited on 25.02.2013
4. Berhane Y, Demissie M. “Cold chain status at immunisation centres in Ethiopia”. East Afr Med J. 2000 Sep;77(9):476-9.
5. Grasso M, Ripabelli G, Sammarco ML, Manfredi Selvaggi TM, Quaranta A. “Vaccine storage in the community: a study in central Italy”. Bull World Health Organ. 1999; 77(4):352-5.
6. Pai HH, Ko YC. “Vaccine storage practices in primary care physicians' offices in Taiwan” Kaohsiung J Med Sci. 1999 May;15(5):274-9.
7. Thakker Y, Woods S. “Storage of vaccines in the community: weak link in the cold chain?” BMJ. 1992 Mar 21;304(6829):756-8.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License