IJCRR - 5(6), March, 2013
Pages: 59-68
Date of Publication: 30-Mar-2013
Print Article
Download XML Download PDF
HOMOCYSTEINE, C-REACTIVE PROTEIN AND TRADITIONAL CARDIOVASCULAR RISK MARKERS IN POLYCYSTIC OVARY SYNDROME
Author: Seerla Lalitha Devi, Syed Abdul Jaweed
Category: Healthcare
Abstract:Objectives: Polycystic ovary syndrome one of the major Endocrine and reproductive abnormality which represent the largest unique young women at high risk for development of premature atherosclerotic heart disease. The Metabolic disturbances associated with PCOS like Insulin resistance, hyperandrogenism, and hypertension may adversely accelerate the cardiovascular risk profile in these young Women. With this background, a case control study was undertaken to evaluate the levels of Traditional and Novel CV risk markers in PCOS women compared to healthy controls. Methods: Fasting blood Glucose, Insulin, Insulin resistance (homeostasis model assessment, HOMA-IR), Testosterone, Lipid levels, Homocysteine, C- reactive protein and Uric acid levels were determined in Fourty diagnosed PCOS women and healthy age matched controls. All biochemical analysis was done using commercial enzymatic kits. Results: Testosterone the main component of PCOS, HOMA-IR were significantly (p< 0.05) higher in PCOS women than controls. Insulin, Total cholesterol, TGL, HDL, LDL levels were also significantly (p< 0.05) higher in PCOS women and the Novel CV markers Homocysteine, CRP, Uric acid were significantly (p< 0.05) elevated in PCOS women compared to controls. Conclusion: Both Traditional and Novel CV risk markers were significantly elevated in PCOS women indicating early onset cardiovascular risk in these young women. Hence, correction and routine screening for these parameters helps in early identification of cardiovascular risk and can prevent the development of endothelial dysfunction, which is a reversible early event in atherosclerosis and appropriate treatment should be aimed to control these parameters.
Keywords: Insulin resistance, Dyslipidemia, Endothelial dysfunction, Cardiovascular risk
Full Text:
INTRODUCTION
Women with Polycystic ovary syndrome (PCOS) constitute the largest group at risk for development of cardiovascular diseases (CVD). The characteristic features of PCOS include, oligomenorrhea, hyperandrogenism, hirsutism and irregular menstrual cycle. Clinical androgen excess in women may signal a risk for coronary artery disease 1 . In time the disorder may lead to onset of insulin resistance, early onset of type-2 diabetes mellitus (DM), and CVD. This may be due to probably the result, in part of the metabolic disturbances associated with PCOS. It has been shown that PCOS can cause severe insulin resistance and its secretion disturbances to some extent. Many observations suggest that there is a strong association between menstrual irregularity and insulin resistance among women with PCOS 2 . Although insulin resistance is not a disease, its presence is associated with increased risk of cardiovascular morbidity and mortality and type 2 diabetes 3 Women with PCOS are frequently found to have atherogenic lipid abnormalities that may reflect underlying insulin resistance. Interestingly, it was observed that women with hirsutism and regular cycles do not present dyslipidaemia, whereas those with both hirsutism and oligomenorrhea had lower HDL-C and higher triglycerides, suggesting an association between menstrual irregularity and dyslipidaemia 4 . Women with menstrual irregularities are likely to be those exhibiting more pronounced dyslipidaemia 5 . In recent years, interest has grown in novel biochemical and biophysical markers of cardiovascular risk. C-reactive protein (CRP) has been shown to be a good predictor of vascular events. In addition to being a marker of inflammation, there is evidence that CRP may have a direct role in atherogenesis via adhesion molecule expression, complement activation, and mediation of low density lipoprotein (LDL) uptake by macrophages 6 . Endothelial function and vascular function is also altered in women with PCOS and vascular compliance has been reported to be decreased. Homocysteine is postulated to damage the vascular endothelium directly and also raised homocysteine concentrations have been associated with an increased risk of ischemic heart disease and atherosclerosis7 . Uric acid exerts proinflammatory, prooxidant and proliferative actions at the endothelial cell level that may increase cardiovascular risk. The increase in serum uric acid concentrations is related to cardiovascular events in high-risk subjects, yet this relationship is less established in the general population and the possible roles of uric acid as a causal agent or as a mere marker of cardiovascular risk are debated at present 8 . However all of these are surrogate markers and despite their presence, it remains to be proven (prospectively) that women with PCOS are at increased risk for cardiovascular-related morbidity and mortality. Hence, we aimed to study the levels of both Traditional and Novel CV risk markers in PCOS women and compared with age matched controls inorder to predict the early onset of cardiovascular risk in these patients.
MATERIALS AND METHODS
This was a prospective study done in Department of Biochemistry Bidar Institute of Medical Sciences Bidar (Karnataka), India. After informed consent, Fourty women diagnosed to have PCOS by Rotterdam ESHRE/ASRM PCOS group’s revised 2003 criteria with presence of any two of the three criteria were recruited for the study. These criteria were a) Oligo and/ or anovulation with exclusion of related disorders with similar presentation like hypothyroidism (TSH > 5mIU/ml) b) Clinical and / or biochemical signs of hyperandrogensm, c)Polycystic ovaries with exclusion of congenital adrenal hyperplasia androgen secreting tumors. Other exclusion criteria were patients on oral contraceptives, glucocorticoids, antiandrogens, ovulation inducing agents, antidiabetic drugs or antiobesity drugs or other hormonal drugs during the previous 6 months. The critreria for healthy control group include no menstrual irregularities, hirsuitism, hypertension, Hyperandrogenism and no ultrasound (or) clinical signs of PCOS. None of the subjects were Diabetic, and with acute infections. A family history of Coronary artery disease, smoking, or Concurrent oestrogen, antihypertensive, and lipid lowering medication Subjects were excluded.
Sampling and Storage:
After overnight fasting venous blood samples were collected in a plain bulb, centrifuged at 2000 rpm for 15 minutes and stored immediately at -80 oC until analysis. 1ml of blood was collected in anticoagulated bulb and plasma collected for analysis of glucose.
Biochemical Analysis:
Serum glucose was measured by using glucose oxidase-peroxidase technique. Lipid analysis in fasting serum was performed for all patients. The lipid profile included measurement of the levels of total cholesterol, HDL, LDL and triglyceride. The lipid levels were estimated by commercially available enzymatic assay kits and were expressed as mg/dl. CRP measurements were determined by highly sensitive CRP (hs-CRP) analyzed by immunoturbidometric method using commercial kits. The Homocysteine concentrations were measured by using commercial kit. Analysis of parameters was done on Beckman CX9 auto analyzer using commercial kits. Testosterone levels were analyzed by Chemiluminescence’s method in LIAISON analyzer. Insulin levels were measured by Enzyme linked immunosorbent assay (ELISA) technique and were expressed as µIU/ml. Insulin resistance was determined by the homeostasis model assessment (HOMA- IR)

All subjects had undergone anthropometric measurements including Height (m), weight (kg), measurements were used to calculate the body mass index (BMI = Wt / height in m2 ), evaluation of systolic and diastolic blood pressures. Statistical Analysis: The statistical Package for the Social Sciences (SPSS version 11.5 for Windows) was used for statistical analysis. Results were expressed as mean ± SD. Unpaired t-test (one tailed) was used to compare the means, and a P value less than0.05 was considered to be statistically significant.
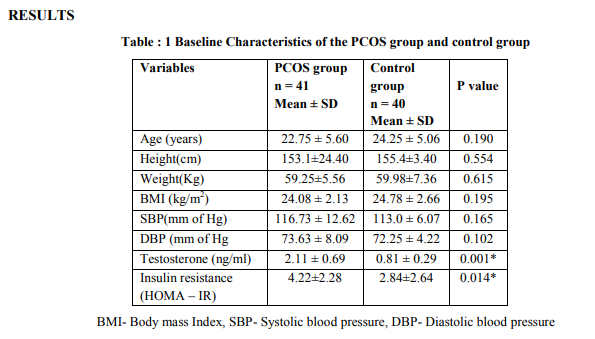
Table: 1 Shows the Baseline characteristics of the Study group and control group. By design, there were no significant differences between the two groups in Age, height, weight and BMI. Similarly, there were no significant differences in systolic and diastolic blood pressure in PCOS women when compared to the control group. The main component of PCOS i.e., serum testosterone levels and Insulin resistance (HOMA-IR) levels were significantly elevated in PCOS women when compared to control group (p =0.000), (p=0.014).
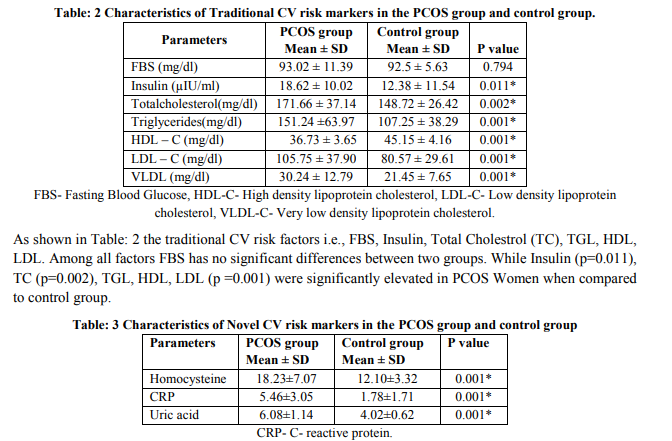
Table: 3 shows the novel cardiovascular risk factors i.e., CRP, Homocysteine, and uric acid were found to be significantly elevated in PCOS women (p =0.001) when compared to the control group.
DISCUSSION
This study was attempted to understand whether the traditional and novel CV risk markers were increased in PCOS women in case which may lead to early onset of cardiovascular risk in these population. We noted an abnormal lipid profile, Hyperinsulinemia, Insulin resistance, and increase levels of Homocysteine, CRP, Uric acid levels in PCOS women when compared with age matched controls. It was first reported by Burghen et al 9 in 1980 and subsequently confirmed by Chang et al 10 in 1983 that insulin resistance is present in PCOS women. Insulin resistance is now recognized as a major risk factor for the development of type 2 (non-insulin-dependent) diabetes mellitus (NIDDM). Dunaif et al 11 demonstrated that women with PCOS were insulin resistant, independent of obesity. Many women with PCOS exhibit β-cell dysfunction reducing insulin response to a glucose load insufficient for the degree of insulin resistance. The mechanism responsible includes the markedly diminished insulin-induced receptor autophosphorylation seen in 50% of women with PCOS. This defect is unique to women with PCOS and is attributable to an abnormal protein tyrosine kinase receptor. In the present study the PCOS women showed Hyperinsulinemia (p = 0.011) and insulin resistance. Fasting insulin levels were significantly higher in PCOS group in accordance to previously published studies 12,13, 38. Insulin resistance calculated by HOMA-IR was also found to be elevated in the PCOS group (p = 0.014). Insulin resistance results in hyperandrogenemia due to decrease in sex hormone binding globulin (SHBG). This insulin resistance and the resulting hyperinsulinemia contribute to the reproductive abnormalities of PCOS women 9 . Insulin resistance and androgen excess together collaborate in increasing risk for type 2 DM in PCOS women, a well known risk factor for macrovascular and particularly coronary artery disease. All the PCOS women in the present study had hyperandrogenemia (p=0.001). Polycystic ovary syndrome is an associate with a higher frequency of cardiovascular risk factors. In the present study, PCOS women had increased levels of serum total cholesterol (p=0.002), triglycerides, LDL-C and decreased HDL-C levels (p=0.001) indicating more risk for cardiovascular disease. Our study is in line with Olivier V et al 14, who showed that PCOS women were associated with more pronounced atherogenic lipid profile i.e increased LDL-C and decreased HDL-C levels. In an Indian study done by Anuradha K et al 15, PCOS women also showed increased total cholesterol, triglycerides and LDL-C and decreased HDL-C levels in insulin resistance women compared to insulin sensitive women. Mirjana S et al 16 suggested that PCOS per se without obesity affects insulin secretion and lipid metabolism, mainly triglyceride levels which enhances atherogenic potential in these subjects. In another study, by Haffner SM et al 17 demonstrated that insulin resistance is associated with increased triglycerides and decreased HDL-C levels, which is potent combination that promotes coronary heart disease. Bickerton AST et al 7 did not found any difference in lipid parameters in PCOS women compared with controls their possible explanation is reduced insulin sensitivity. This is supported by the observation that the typical disturbance of lipid parameters seen in PCOS is associated with the presence of insulin resistance 18 . The possible explanation is that Hyperinsulinemia and hyperandrogenemia cause adipocytes to undergo increased catecholamineinduced lipolysis and release of free fatty acids into the circulation. Increased free fatty acids in the liver stimulate secretion of very low-density lipoprotein (VLDL), which ultimately leads to hypertriglyceridemia 19. It has been postulated by Wetterau and coworkers20 that insulin inhibits the expression of the microsomal triglyceride protein, which is responsible for the secretion of apolipoprotein B (apoB) and VLDL. Insulin resistance leads to hepatic overproduction of apoB and VLDL and, ultimately, to hypertriglyceridemia. Atherogenic modifications of LDL cholesterol toward smaller, more dense particles have been demonstrated 21 Androgens, particularly testosterone, may have a role by decreasing lipoprotein lipase activity in abdominal fat cells 22. Hepatic lipase has a role in catabolism of HDL particles, is significantly upregulated in hyperandrogenemia i.e, in PCOS women leading to decreased levels in these women. This is demonstrated by Elbers et al 23 , by supplementation of exogenous androgens in a study of female to male transsexuals. This shows that Traditional CV risk markers are increased in PCOS women which indicate more cardiovascular risk. Plasma homocysteine levels are widely accepted as an independent risk factor for cardiovascular disease. Homocysteine has been reported to promote atherosclerosis by inducing endothelial dysfunction through limited bioavailability of nitric oxide and altered blood vessel elasticity; enhancing the activation of coagulation system and increasing the platelet adhesiveness 24.In the Present study we examined the serum levels of Homocysteine in PCOS women and found elevated levels in PCOS women (P=0.001) when compared to control group. Previous studies had shown that PCOS patients have significantly higher plasma homocysteine concentrations with regardless of BMI i.e., in both lean and obese PCOS patients than control group 25, 26 . Some studies shown that, mean plasma homocysteine levels are significantly higher in Insulin-resistant PCOS patients when compared with non-insulinresistant PCOS patients regardless of BMI which indicates relationship of Homocysteine with IR 27, 28 . Plasma levels of insulin seem to influence homocysteine metabolism through effects on glomerular filtration or by influencing activity of some important enzymes in homocysteine metabolism like methyltetrahydrofolate reductase (MTHFR) and hepatic cystathione β synthase (CBS). Homocysteine levels are seems to be related with risk of cardiovascular disease and complications, by increasing oxidative stress in vascular endothelium, activation of platelets impairment of blood flow stimulation of vascular smooth muscle proliferation and may be one of the signals inducing apoptosis in vascular endothelial cells by activating unfolded protein response 29, 30. Homocysteine levels are influenced by a number of variables, including smoking, renal function, vitamin B12, folate status and enzyme dysfunction states. All of the patients in our study are nonsmokers. Their renal function was normal as evident from the normal serum creatinine levels (0.7?0.052 mg/dl). Methyltetrahydrofolate reductase (MTHFR) enzyme deficiencies and vitamin levels were not screened in this patient group, as in the study by Laivuori et al. 31 and Tsanadis et al. 32 who had studied the enzyme levels and did not found significant levels. Vitamin B12 levels and folic acid levels were examined in the study by Yarali et al 25 and no significant differences were found between PCOS and controls. The vascular endothelial aspect and Hyperinsulinemia might be responsible for the higher homocysteine levels in these patients. Serum markers of inflammation are being increasingly recognized as predictors of atherosclerosis and cardiovascular diseases. Chronic inflammation results in endothelial dysfunction and facilitates the initiation of an atherosclerotic process. Several studies suggest that low grade inflammation, reflected by elevated C-reactive protein (CRP), can contribute to the development of atheriosclerosis 33. CRP is considered not only an inflammatory marker of atherosclerosis but also a mediator of the disease because it contributes to the pathogenesis of lesion formation by interacting with the endothelium and therefore CRP can be seen as a measure of endothelial dysfunction 34. CRP can independently predict type 2 diabetes and has been linked to insulin resistance 35. However, the role of inflammation in the etiology of cardiovascular disease (CVD) and other metabolic diseases is still disputed and is not generally accepted. In our study we find elevated hs-CRP (p=0.001) levels when compared with age matched controls. Our findings are in agreement with other studies who has found a significant difference in CRP levels between PCOS patients and controls, and they suggested a chronic subclinical inflammatory process may be the possible underlying mechanism of atherosclerosis in some PCOS patients 36, 37 . Recent evidence suggests that CRP may have a direct role in the pathogenesis of atherosclerotic lesion formation and appears to affect a number of interrelated pathways in the vascular endothelium, including the induction of expression of adhesion molecules, foam cell formation by inducing LDL opsonisation and which cause monocyte recruitment in the arterial wall. CRP also activates complement and endothelial cell (EC) sensitization which leads to EC damage finally leading to atherosclerotic plaque rupture 38. Theoretically, CRP may differentiate between those PCOS women who are at high risk for developing Type-2 Diabetes and CVD 39 . Uric acid is another newly described coronary risk factor. PCOS women having insulin resistance may lead to hyperuricemia. Recently, an inverse correlation between serum uric acid concentrations and insulin sensitivity has been described in subjects with varying degrees of metabolic syndrome, suggesting that measurement of serum uric acid may provide a simple marker of insulin resistance 40 . In the present study high uric acid levels (p=0.001) were seen in PCOS women compared to control group. This is in contrast to Manuel LuqueRam?´rez1, et al 8 , who found no significant differences in uric acid levels between PCOS women and non-hyperandrogenic control women. Similarly Anttila L et al 40 also found no differences in uric acid concentrations between PCOS women and control group. The findings of the present study are in contrast to the studies described above. Uric acid levels are affected by ethnicity, BMI, renal function. Obesity is the main determinant of serum uric acid levels in PCOS patients 8 . However, obesity cannot be the contributing factor in the present study because the study group and control group were BMI matched to remove the influence of BMI. PCOS women are insulin resistant and exhibit hyperinsulinism. Hyperinsulinism has an inhibitory effect on the renal excretion of uric acid which might explain the higher uric acid levels in PCOS women, though in the reference range compared to the control group. The elevated uric acid levels might be due to Androgens which may increase serum uric acid levels by inducing the hepatic metabolism of Purines 8 . This is supported by the finding that only the antiandrogenic oral contraceptive pill Diane35 Diario, and not the insulin sensitizer metformin, decreased uric acid concentrations significantly in PCOS women. Increases in uric acid concentrations as small as 59 ?mol / strangulated hernias L (0.99mg/dl), increase the frequency of cardiovascular events and ischemic cardiopathy 8 .
CONCLUSION
In conclusion the findings of the present study show significantly elevated levels of both Traditional and Novel Cardiovascular risk markers in PCOS women. The altered glucose metabolism is due to pathogenesis of insulin resistance and abnormal lipid profile results in increased risk for cardiovascular morbidity in PCOS Patients. At the same time, inflammatory risk marker CRP, homocysteine can cause endothelial dysfunction and thus increase the risk of early onset cardiovascular risk in these young women. Hence, correction of these CV risk factors in PCOS women can play an important role in decreasing the cardiovascular mortality in these patients. Hence, routine screening for these parameters helps in early identification of these cardiovascular risk factors can prevent the development of endothelial dysfunction, which is a reversible early event in atherosclerosis and appropriate treatment should be aimed to control these parameter.
ACKNOWLEDGEMENTS
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to Authors / editors/ publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
1. Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Clin Obstet Gynaecol. 2004; 18 : 671-683.
2. Abbott DH, Dumesic DA, Franks S. Developmental origin of polycystic ovary syndrome - a hypothesis. J Endocrinol 2002; 174: 1-5.
3. Reaven G. Metabolic syndrome: pathophysiology and implications for management of cardiovascular disease. Circulation 2002; 106: 286-288.
4. Taponen S, Martikainen H, Jarvelin MR, et al. Metabolic cardiovascular disease risk factors in women with self reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland Birth Cohort 1966 Study. Clin Endocrinol Metab 2004; 89: 2114-2118.
5. Eleni Kousta, George Tolis, Stephen Franks. Polycystic ovary syndrome. Revised diagnostic criteria and long-term health consequences Hormones 2005; 4(3):133-147.
6. Blake GJ, Ridker PM. Inflammatory biomarkers and cardiovascular risk prediction. J Intern Med 2002; 252:283–94.
7. Bickerton A S T, Clark N, Meeking D, Shaw K M, Crook M, Lumb P, et al. Cardiovascular risk in women with polycystic ovarian syndrome. J Clin Pathol 2005; 58:151–154.
8. Manuel Luque-Rami´rez, Francisco A lvarezBlasco, Miguel Giovanni Uriol Riveraand He´ctor F. Escobar-Morreal. Serum uric acid concentration as non-classic cardiovascular risk factor in women with polycystic ovary syndrome: effect of treatment with ethinylestradiol plus cyproterone acetate versus metformin. Human Reproduction 2008; 23(7): 1594–1601.
9. Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab 1980;50:113-116.
10. Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in non obese patients with polycystic ovarian disease. J Clin Endocrinol Metab 1983; 57: 356-359.
11. Dunaif A, Finegood DT. β-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab 1996;81:942-947.
12. Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome. J Clin Endocrinol Metab 1998; 83:2694-2698.
13. Hemati T, Moghadami-Tabrizi N, DavariTanha F, Salmanian B, Javadian P. High plasma homocysteine and insulin resistance in patients with polycystic ovarian syndrome. Iranian Journal of Reproductive Medicine 2011; 9 :223-228.
14. Olivier V, Regine P, Steegers-Theunissen M, Huberdina P, Smedts M, Geesje M. A more atherogenic serum Lipoprotein profile is present in women with polycystic ovary syndrome: A case-control study. J Clin Endocrinol Metab 2008;93(2):470-476.
15. Anuradha K, Sreekumari N, Lavanya R. Association of obesity and Insulin resistance with dyslipidemia in Indian women with polycystic ovary syndrome. Ind J Med Scien 2006;60;11:447-453.
16. Mirjana. S, Milos Z, Jasmina C. Insulin levels and lipid profile in lean women with polycystic ovary syndrome. Abstracts 2007;14:649.
17. Haffner SM, Valdez RA, Hazude HP, Mitchel BD, Morales PA, Stern MP et al. Prospective analysis of the insulin-resistance syndrome (syndrome x). Diabetes 1992;41:715.
18. Robinson S, Henderson AD, Gelding SV, et al. Dyslipidaemia is associated with insulin resistance in women with polycystic ovary syndrome. Clin Endocrinol 1996;44:277–84.
19. Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, Chrousos P. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab 2007;18:280-285.
20. Wetterau JR, Lin MC, Jamil H. Microsomal triglyceride transfer protein. Biochem Biophys Acta 1997;1345:136-150.
21. Dejager S, Pichard C, Giral P, et al. Smaller LDL particle size in women with polycystic ovary syndrome compared to controls. Clin Endocrinol (Oxf) 2001;54:455-462.
22. Polderman KH, Gooren JG, Asscherman H. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab 1994;79:265-271.
23. Elbers JM, Giltay EJ, Teerlink T, Scheffer PG, Asscheman H, Seidell JC et al. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol (oxf) 2003;58:562-571
24. Hanratty CG, McGrath LT, McAuley DF, Young IS, Johnston GD. The effects of oral methionine and homocysteine on endothelial function. Heart 2001; 85(3):326-30.
25. Yarali H, Yildirir A, Aybar F, Kabakci G, Bukulmez O, Akgul E, Oto A.Diastolic dysfunction and increased serum homocysteine concentrations may contribute to increased cardiovascular risk in patients with polycystic ovary syndrome. Fertil Steril 2001;76(3): 511-6.
26. Loverro G, Lorusso F, Mei L, Depalo R, Cormio G, Selvaggi L. The plasma homocysteine levels are increased in polycystic ovary syndrome. Gynecol Obstet Invest 2002;53(3): 157-62.
27. Morey Schachter, Arieh Raziel, Shevach Friedler, Deborah Strassburger, Orna Bern and Raphael Ron-El, Insulin resistance in patients with polycystic ovary syndrome is associated with elevated plasma homocysteine. Human Reproduction 2003; 18(4):721-727.
28. Ilhan Tarkun, Berrin Cetinarslan Zeynep, Canturk Erdem Turemen. The Plasma Homocysteine Concentrations and Relationship with Insulin Resistance in Young Women with Polycystic Ovary Syndrome Turkish. Journal of Endocrinology and Metabolism 2005; 1: 23-28.
29. Mujumdar, V.S., Tummalapalli, C.M., Aru, G.M. and Tyagi, S.C. Mechanism of constrictive vascular remodeling by homocysteine: role of PPAR. Am. J. Physiol 2002;282:1009-10015.
30. Zhang, C., Yong, C., Adachi, M.T., Oshiro, S., Aso, T., Kaufman, R.J. and Kitajima, S. Homocysteine induces programmed cell death in human vascular endothelial cells through activation of the unfolded protein response. J. Biol. Chem 2001;276:35867- 35874.
31. Laivuori, H., Kaaja, R., Turpeinen, U., Viinikka, L. and Ylikorkala, O. Plasma homocysteine levels elevated and inversely related to insulin sensitivity in preeclampsia. Obstet. Gynecol 1999;93:489-493.
32. Tsanadis, G., Vartholomatos, G., Korkontzelos, I., Avgoustatos, F., Kakosimos, G., Sotiriadis, A., Tatsoni, A., Eleftheriou, A. and Lolis, D. Polycystic ovarian syndrome and thrombophilia. Hum. Reprod 2002; 17: 314-319.
33. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women.N Engl J Med 2000; 342: 836-843.
34. Verma S, Buchanan MR, Anderson TJ. Endothelial function testing as a biomarker of vascular disease. Circulation 2003; 108: 2054-2059.
35. Freeman DJ, Norrie J, Caslake MJ, et al. West of Scotland Coronary Prevention Study, C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes 2002; 51: 1596-1600.
36. Boulman N, Levy Y, Leiba R, Shachar S, Linn R, Zinder O, and Blumenfeld Z. Increased C - reactive protein Levels in the Polycystic Ovary Syndrome: A Marker of Cardiovascular Disease. Journal of Clinical Endocrinology and Metabolism 2004; 89(5):2160–2165.
37. Talbott EO, Zborowski JV, Boudreaux MY, Mchugh-pemu KP, Sutton- tyrrell K, Guzick D.S. The Relationship between C - reactive protein and Carotid Intima-Media Wall Thickness in Middle-Aged Women with Polycystic Ovary Syndrome. The Journal of Clinical Endocrinology and Metabolism 2004; 89(12):6061–6067.
38. Alain Tedgui. The role of inflammation in atherothrombosis: implications for clinical practice. Vascular Medicine 2005; 10: 45–53.
39. Ridker PM, Buring JE, Cook NR, Rifai N. Creactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8- year follow-up of 14719 initially healthy American women. Circulation 2003; 107:391–397.
40. Anttila L, Rouru J, Penttil T, and. Irjala K. Normal serum uric acid concentrations in women with polycystic ovary syndrome. Human Reproduction 1996; 11: 2405-2407.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License