IJCRR - 5(4), February, 2013
Pages: 73-81
Date of Publication: 28-Feb-2013
Print Article
Download XML Download PDF
DO STATINS HAVE POTENTIAL AS ANTI OSTEOPOROTIC DRUGS AND CAN THEY BE USED FOR PREVENTION OR TARGETING OSTEOPOROSIS: A REVIEW
Author: Karmajeet Rath, Biswa Bhusan Mohanty, Sanjay Kumar, Pramila Nayak, Jagannath Sahoo
Category: Healthcare
Abstract:Inhibitors of HMG-CoA reductase enzymes, which are statins, are well known and much prescribed drugs for lowering of cholesterol and used for dyslipidemia. But, they have also been shown across various studies to stimulate bone formation. In bone cells, these inhibitors have been known to increase the gene expression of bone morphogenetic protein-2, thereby helping in osteoblastic differentiation, with some effects also on osteoclastic inhibition. The findings that statins can increase bone formation and thereby mass of bone, can be helpful in preventing bone fractures and improving osteoporosis, which is a condition of marked bone loss. Hence, it is reviewed herein that HMG-CoA reductase inhibitors can be a great way, probably a futuristic drug for osteoporotic treatment and prevention. Keywords: HMG-CoA reductase inhibitors, osteoporosis
Keywords: HMG-CoA reductase inhibitors, osteoporosis, osteoblasts, statins, bone metabolism, fracture
Full Text:
INTRODUCTION
There has been a remarkable increase in the knowledge about osteoporosis since the past 25 years or so. Osteoporosis is seen to occur more in females than in males, although mortality is higher in men, which are caused by osteoporotic fractures 1, 2, 3. Added to this, post menopausal women have a higher frequency of osteoporotic fractures and osteoporotic incidences 4 . Bone is a metabolically active organ in which mineral and organic components will be important to determine the mechanical function of skeleton5,6 . Bone turnover is under the control of certain defined agents as well as processes, which regulate the formation of bones and also resorption of bone. These are two basic but important processes by which the bone remodelling is occurg. New bone formation is basically a function of the osteoblasts, agents which act by increasing or decreasing the replication of cells in the lineage of osteoblasts, or modifying the differentiated function of the osteoblast. It would therefore be beneficial for stimulating the osteoblastic activity at local sites in bone by an oral anabolic agent, resulting in bone formation, where and when needed. Osteoporosis is defined clinically as reduced bone mass, to such a level where it will result in fracture, with minimal trauma7 . This term will also suggest that there is parallel loss of both bone mineral and matrix that will render the mineralised bones incapable of withstanding minor trauma, without leading to fractures. Osteoporosis is a disease condition which is affecting about 30 million people in United States and about 100 million people worldwide. Unless the bone mass falls below 30% to 50% lower than the normal value, fractures do not occur.
DYNAMICS OF BONE & OSTEOPOROSIS
Bone mass is the net result of a balance between bone formation and resorption. This balance is mostly regulated by genetic as well as environmental factors. Genes regulating bone mass are vitamin D receptor gene, estrogen receptor gene, interleukin-6, transforming growth factor B and gene encoding type 1 collagen 8,9,10,11. Environmental factors include nutritional status, exercise, drugs like glucocorticoids and contraceptive pills, neoplastic diseases such as myeloma, leukemia, lactation, parity, alcohol, smoking, weight loss and long hip axis length. Although the exact pathophysiology of osteoporosis is unknown, an imbalance between bone formation and resorption presumably causes bone mass to decline in adulthood and osteoporosis occurs when the amount of bone removed from the skeleton by bone resorbing osteoclasts exceeds the amount formed by osteoblasts during the coupled process of remodelling. Treatment of osteoporosis is aimed towards restoring this balance.
BONE REMODELLING
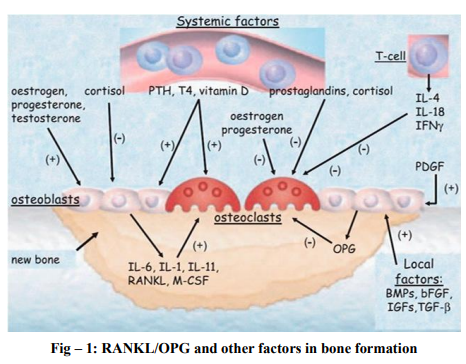
Bone remodelling is well established throughout the literature and involves both systemic and non systemic factors. It is well known that in this procedure an important role is played by the system of receptor activator of nuclear factor kappa b ligand – osteoprotegerin 6, some cytokines and bone morphogenic proteins. Mesenchymal stem cells are pluripotent cells with a high mitotic index and are involved in the differentiation of adipocytes under the regulation of genes and transcription factors. Adipose tissue is considered as a separate endocrine gland, responsible for the secretion of adipokines such as leptin, adiponectin and hormones such as vitamin D3, estrogen, etc and in involved in the pathogenesis of some entities. Leptin is considered to exert a control over the RANKL/OPG axis by decreasing the expression of RANKL and increasing OPG to bring out preosteoblasts and mononuclear cells into the circulation. 12,13. There is diversion of adipocyte into an osteoblast, which is a multifactorial process regulated by various factors.
Molecular biology and genetics reveal that both vascular and osteoblast biology have a common pathway of RANK/RANKL/OPG 14. Mundy and colleagues in 1999 reported first that there was an anabolic effect of statins in human bone cells and cultured mouse. Simvastatin and lovastatin enhanced the expression of bone morphogenetic protein- mRNA15 . Statins as potential drugs for osteoblastic activites Several experiments have illustrated the effect of statins in bone metabolism in vitro and vivo. Chan et.al.16 in 2000 demonstrated that there was negative effect of statin on bone repair. But in contrast, there were many other studies which showed beneficial effects. The administration of statins has anabolic effects on the bone by suppressing osteoclasts and promoting osteoblastic activities. Hence, statins can effectively work on bone formation and increase the bone mass density by inhibiting it, thereby helping in prevention of osteoporosis and aiding in fracture healing. Most of these studies have been done in different doses in animal models, apart from showing actions of cholesterol lowering in animals. Some also have shown remarkable increase in required growth factors such as TGFb-1 and VGF or Vascular Growth Factor, possibly showing a path for the statins and bone interaction. There have been more studies in cell cultures, in vitro, which support the findings that statins have a potential mechanism on bone metabolism. There is expression in certain genes like BMP-2, COLLIA1, Osteocalcin, etc while the RANKL gene is depressed are affected by statins, which might be the reason for the bone formation. In 2007, Hughes A et.al. 17 found that the statins of hydrophobic nature and hydrophilic nature have inhibited osteoclastic action in vitro, while some other studies have shown lipophilic agents like simvastatin to have better action 18. It was the pleiotropic effect of statins which led many clinicians to study the use of statins in bone metabolism. A meta analysis by Uzzan et al 19 , found that statins have a positive effect on bone mass density in different bones of the body. It was concluded that there was statistically significant but modest positive effect of statins on BMD. More data is still needed to support the use of statins in prevention of bone fracture. Most of the literatures showed an increase in BMD and also in bone markers. All the available data from various literatures, starting from the experimental studies to the observational studies showed that there is surely some positive effect of statins on BMD. One meta analysis by Bauer et al 20 also showed the beneficial effect of statins on fracture risk. It was demonstrated by Chuengsamarn et al 21 that statins could inhibit bone resorption and stimulate bone formation, with a dual action on bone metabolism. It might be possible for statins to gain a position as a drug used for prevention and management of osteoporosis, so much so that statins is being frequently prescribed by clinicians for other treatments. Possibly in the future, drugs will be up in circulation, which will target the different pathophysiological and biochemical cascades as in statins with alterations of doses and could be used for different bone disorders like osteoporosis.
DISCUSSION
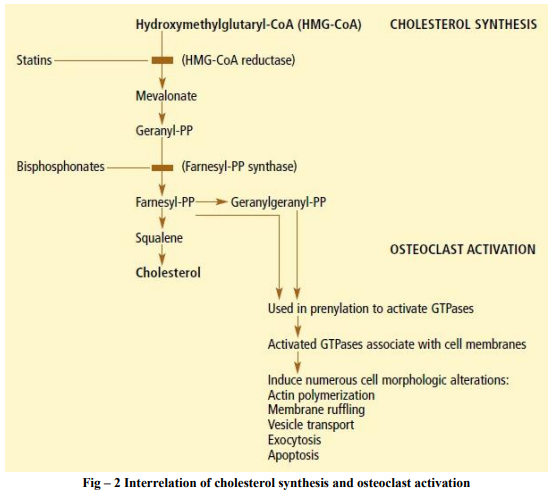
Two recently elucidated pathways may explain how statins can be helpful in building up bone. The first mechanism is through the inhibition of mevalonate production. Many laboratories and researchers noted that cholesterol synthesis and osteoclast activation involved same biochemical cascade. The synthesis of cholesterol is done in plenty of steps. HMG-CoA is converted into mevalonate by enzyme HMG-CoA reductase. This enzyme is inhibited by statins. Then the mevalonate is converted to geranyl phosphate, which is in turn converted to farnesyl pyrophosphate by the enzyme farnesyl pyrophosphate synthese, which is inhibited by bisphosphonate drugs. Then the cholesterol is synthesised22, 23,24. Osteoclasts use the intermediate molecules of farnesyl pyrophosphate and geranyl pyrophosphate from farnesyl pyrophosphate to modify and activate the key intracellular proteins glutamyl transpeptidases and GTP-ase in a process called prenylation25. Bisphosphonates like alendronate and risedronate inhibit farnesyl synthase, preventing the formation of lipid products 26 .
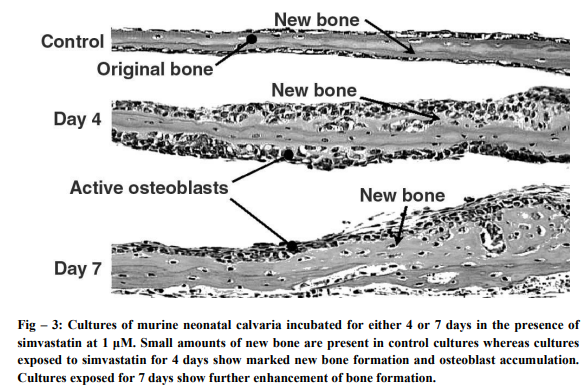
Statins are as efficacious in not allowing osteoclast activation, even in experimental set ups, the action being due to mevalonate production. This will reduce the bone resorption and there is restoration of bone resorption and formation. In many clinical trials, it has been noted that fragility related fractures are reduced27, 28, and 29 . B. Another mechanism which has statins affecting skeleton is that of activation of bone morphogenetic protein-2 promoter. This is a kind of protein which is a growth factor, which leads the osteoblasts into proliferating, maturing and thereby creating a new bone30. In an experiment, when lovastatin was injected into organ cultures of calvarial bones from the neonate mice, thrice a day for 5 days, volume of bone increased by as much as 50%, in comparison to placebo. Histological studies showed enhanced bone formation and osteoid accumulation. 31 .
Although a number of recent drugs have shown to prevent osteoporosis and have been used in the treatment, not one of them has been shown to stimulate bone formation and increase osteoblasts activity. It was suggested that drugs which inhibit HMG CoA reductase, which are statins, many have such effects and reduce the risk of osteoporotic fractures. 30. Many other reports have also suggested such an anabolic bone effect, which might show a new path towards the treatment of osteoporosis. 32. To aid in the proof of such an action, it was stated that statins as a class of unknown bone anabolic agents acted through increased production of one of the important bone growth factors, which is Bone Morphogenetic Protein-2, which directly stimulates the osteoblastic activity and bone formation. Lovastatin was found to be stimulating the BMP-2 promoter, from among 30,000 natural compounds tested. 33. Many other subsequent studies have also demonstrated similar effects through fluvastatin, simvastatin and mevastatin. 34, 35, 36. These studies showed that the drugs inhibiting HMG-CoA reductase commonly utilised nowadays for lowering blood lipid levels, also stimulate bone formation activity by osteoclasts through the increase of BMP-2 expression, which is a proven differentiator of osteoblasts. Chuengsamarn et al also carried out many experiments in form of prospective randomised trials on the impact of simvastatin on osteopenic patients and found that bone formation marker and BMD was significantly higher than in the statin group and the difference of bone resorption market was also significantly lower than in the statin group. 37 .
CONCLUSION
From such studies it can be very well concluded that simvastatin as well as other drugs in this particular group act as double therapeutic weapon by inhibiting the conversion of HMGCoA to mevalonate, which is required for cholesterol synthesis and also inhibits the stimulation of osteoclastic activity. Also, there is increase in the stimulation of osteoblastic activities, providing enough evidence that the simvastatin has a promising role in fighting against osteoporosis. But still more studies are required to find the particular doses and the effect dosage and mode of administration of these statin group of drugs to make it commercially viable. Current therapies of osteoporosis treatment include estrogen replacement therapy, bisphosphonates and selective estrogen receptor modulators and all of them aim at blunting the resorption of bone remodelling. Based on previous findings it was found by Bauer and Cummings that large databases showed association between statin usage and skeletal status, showing relationship between statin use, bone mineral density and subsequent fractures. 38,39. Even a study on post menopausal women has come out which shows an increase in the bone mineral density who are taking statins40. It has been shown to have a protective effect against non pathological fractures among older women. 41,42,43,44 A similar story was involved with the use of Hormone Replacement Therapy or HRT, which was prescribed to ladies around 2000, without much long term studies. Statins are also being pushed nowadays for prevention of fractures. But, with the proper tests and long term studies, it will be coming out clearly, whether the benefits are worth making public.
ACKNOWLEDGEMENTS
Authors acknowledge the great help received from the scholars whose articles cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. Authors are grateful to IJCRR editorial board members and IJCRR team of reviewers who have helped to bring quality to this manuscript.
References:
1. Data from the World Health Organization Assessment of osteoporosis at the primary health care level. Summary report of a WHO scientific Group 2007; WHO, Geneva.
2. Cole ZA, Dennison EM, Cooper C, 2008 Osteoporosis epidemiology update. Curr Rheumatol Rep 10: 92-96.
3. Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV, 2007 Residual lifetime risk of fractures in women and men. J Bone Miner Res 22: 781-788.
4. Pinheiro MM, Reis Neto ET, Machado FS, et al, 2010 Risk factors for osteoporotic fractures and low bone density in pre and postmenopausal women. Rev Saude Publica 44: 479-485.
5. Glimcher MJ, Krane SM: The organizational and structure of bone and the mechanism of calcification. In: A treatise on collagen.Biology of collagens. Edited by Ramachandran GN, Goudl BS. New York: Academic Press; 1968:68-91.
6. Glimcher MJ: Comparison, structure and organization of bone and other mineralized tissues and the mechanism of calcification. In: Handbook of physiology, endocrinology, parathyroid gland. Edited by Aubarch GD. Washington: American Physiological Society; 1976:25-48
7. Al-Sebaie H, Al-Hefnawy A. Risk factors for osteoporosis. Egypt Rheum 1997; 24:209-219
8. Morrison N, Qi J, Tikota A, Kelly PJ, Crofts L, Nguyen TV, et al. Prediction of bone density for vitamin D receptor alleles. Nature 1994; 367:384-387.
9. Stuart H, Ralston M. What determines peak bone mass and bone loss. In: Bailliers Clinical Rheumatology, 3rd ed. Philadelphia: Catham Company 1997; 11:479-493.
10. Girasol G, Jjlka R, Passeri G. Estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells. J Clin Invset 1992; 89:883-891.
11. Grant S, Reid D, Blak G, Herd R, Fogelman I, Ralston SH. Reduced bone density and osteoporosis associated with a polymorphic site in the collagen type 1 alpha gene. Nature Genetics 1996; 14:203-205.
12. Vega D, Maalouf NM, Sakhaee K, 2007 Clinical review: the role of receptor activator of nuclear factor kappa B(RANK)/RANK ligand/osteoprotegerin clinical implications. J Clin Endocrinol Metab 92: 4514-45121.
13. Painter SE, Kleerekoper M, Camacho PM, 2006 Secondary osteoporosis: a review of the recent evidence. Endocr Pract 12: 436- 445.
14. Bagger YZ, Rasmussen HB, Alexandersen P, et al, 2007 PERF Study Group. Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos Int 18: 505-512.
15. Mundy G, Garrett R, Harris S, et al, 1999 Stimulation of bone formation in vitro and in rodents by statins. Science 286: 1946- 1949.
16. Chan KA, Andrade SE, Boles M, Buist DS, Chase GA,Donahue JG, et al. Inhibitors of hydroxymethylglutaryl-CoA reductase and risk of fracture among older women. Lancet 2000;355:2185-8.
17. Hughes A, Rogers MJ, Idris AI, et al, 2007 A Comparison between the Effects of Hydrophobic andHydrophilic Statins on Osteoclast Function In Vitro and Ovariectomy-Induced Bone Loss In Vivo. Calcif Tissue Int 81:403-413.
18. Pagkalos J, Cha JM, Kang Y, et al, 2010 Simvastatin induces osteogenic differentiation of murine embryonic stem cells. J Bone Miner Res, 25: 2470-2478.
19. Uzzan B, Cohen R, Nicolas P, Cucheratc M, Perret G. Effects of statins on bone mineral density: a meta-analysis of clinical studies. Bone 2007; 40:1581-1587.
20. Bauer DC, Mundy GR, Jamal SA, Black DM, Cauley JA, Harris F,Duong T, Cummings SR: Statin use, bone mass and fracture: an analysis of two prospective studies. J Bone Min Res 1999, 14:1188.
21. Chuengsamarn S, Rattanamongkoulgul S, Suwanwalaikorn S, Wattanasirichaigoon S, Kaufman L. Effects of statins vs. non-statin lipid-lowering therapy on bone formation and bone mineral density biomarkers in patients with hyperlipidemia. Bone 2010;46:1011-1015
22. Fisher JE, Rogers Mi, Halasy JM, Luckman SP, Hughes DE, Masarachia† PJ, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci USA 1999; 96:133 138.
23. Luckman SP, Hughes DE, Coxon FP, Russell RG, Rogers MJ. Nitrogencontaining bisphosphonates inhibit the mevalonate pathway and prevent posttranslational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 1996; 13:581-589.
24. Luckman SP, Coxon FR, Ebetino FH, Russell RG, Rogers MJ. Heterocyclecontaining bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenylation: evidence from structure-activity relationships in 1774 macrophages. J Bone Miner Res 1998; 13:1668-1678.
25. Zhang FL Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem 1996; 65:241-269.
26. Van Beek E, Pieterman E, Cohen L, Lowik C, Papapoulos S. Farnesyl pyrophosphate synthase is the molecular target of nitrogencontaining biaphosphonates. Biochem Biophys Res Commun 1999; 264:108-211.
27. McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 2001; 344:333- 340.
28. Karpf DB, Shapiro DR, Seeman E, Ensrud KE, Johnston CC Jr, Adami S, et al. Prevention of nonvertebral fractures by alendronate: a meta-analysis. Alendronate Osteoporosis Treatment Study Group. JAMA 1997; 277:1159-1164
29. Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1996; 280:2077-2082
30. Mundy C, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science 1999; 266:1946-1949.
31. Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, et al. Mevinolin: a highly potent competitive inhibitor of hydroxymethyl-glutaryl - coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci USA 1980; 77:3957- 3961
32. Haffner SM, Alexander CM, Cook TJ, Boccuzzi SJ, Musliner TA, Pedersen TR, et al. Reduced coronary events in simvastatin treated patients with coronary heart disease and diabetes or impaired fasting glucose levels, subgroup analyses in the Scandinavian Simvastatin Survival Study. Arch Intern Med 1999; 159:2661-2667
33. Crisby M, Fredriksson-Norden G, Nillsson J. Pravastatin treatment decreases lipid content and cell death in human carotid plaques. Lancet 1998; 30:21-28.
34. Wai-Keilam C, Chan IH, Mundy G. Statins and bone formation. Science 2001; 31:27- 29.
35. Mesako S, Kodama. T, Konishi K. Compactin and simvastatin, but not pravastatin, induce bone morphogenetic protein-2 in human osteosacoma cells. Biochern Biophys Res Commun 2000; 271:688-6
36. Wang PS, Solomon DH, Morgan H, Avorn J. HMG-CoA reductase inhibitors and the risk of hip fractures in elderly patients. JAMA 2000; 283:3211-3216
37. Chuengsamarn S, Rattanamongkoulgul S, Suwanwalaikorn S, Wattanasirichaigoon S, Kaufman L. Effects of statins vs. non-statin lipid-lowering therapy on bone formation and bone mineral density biomarkers in patients with hyperlipidemia. Bone 2010;46:1011-1015
38. Maeda T, Matsunuma A, Kawane T, Horiuchi N: Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun 2001, 280: 874-877.
39. Bauer DC, Mundy GR, Jamal SA, Black DM, Cauley JA, Harris F, Duong T, Cummings SR: Statin use, bone mass and fracture: an analysis of two prospective studies. J Bone Min Res 1999, 14:1188
40. Edwards CJ, Hart DJ, Spector TD: Oral statins and increased bone-mineral density in postmenopausal women. Lancet 2000, 355:2218-2219.
41. Wang PS, Solomon DH, Mogun H, Avorn J: HMG-CoA reductase inhibitors and the risk of hip fractures in elderly patients. JAMA 2000, 283:3211-3216.24.
42. Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H: Statin drugs and the risk of fracture. JAMA 2000, 284:1921-1922,)
43. van Staa TP, Wegman SLJ, de Vries F, Leufkens HGM, Cooper C: Use of statins and risk of fractures [abstract 1067]. J BoneMin Res 2000, 15(suppl):s155.
44. Cauley JA, Jackson R, Pettinger M, Lacroix A, Bauer D, Chen Z, Daugherty S, Hsia J, Lewis CE, McGowan J, McNeeley SG, Passaro M: Statin use and bone mineral density (BMD) in older women: The Women’s Health Initiative Observational Study (WH I-OS) [abstract 1068]. J Bone Min Res 2000,15(suppl):s155.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License