IJCRR - 5(4), February, 2013
Pages: 01-09
Date of Publication: 28-Feb-2013
Print Article
Download XML Download PDF
ACCUMULATION OF HEAVY METALS BY PLEUROTUS OSTREATUS FROM SOILS OF METAL SCRAP SITES
Author: G.A. Boamponsem, A.K. Obeng, M. Osei-Kwateng, A.O. Badu
Category: General Sciences
Abstract:Heavy metal contamination of our ecosystem is one of the major environmental challenges facing our world today. Fungi have the ability to take out heavy metals from soils. Studies were conducted to determine the effectiveness of using Pleurotus ostreatus for the removal of copper (Cu), zinc (Zn), manganese (Mn) and iron (Fe) from soils of metal scrap sites. P. ostreatus was cultivated on soil samples using sawdust as a substrate. With soils of metal scrap sites, Fe recorded the highest level (10.740 \? 68.500 mg/kg) and the least accumulated metal was Cu with a range of < 0.02\? 26.97 mg/kg. In both harvests, Fe was the most absorbed metal followed by Mn, Zn and Cu. In the first harvest, Fe absorption by P. ostreatus was 2.193 \? 3.233 mg/kg and that of Mn, 0.486 \? 0.580 mg/kg. Zn and Cu concentrations were in the range of 0.276 \? 0.539 mg/kg and 0.0200 \? 0.0290 mg/kg respectively. With the second harvest, levels of Fe and Mn in P. ostreatus ranged from 2.33 \? 3.79 mg/kg and 0.499 \? 0.713 mg/kg respectively. Zn concentration was 0.236 \? 0.565 mg/kg and Cu recorded 0.0165 \? 0.0820 mg/kg. With the control soil, concentrations of heavy metals in P. ostreatus were 0.0110 \? 2.027 mg/kg in first and 0.0110 \? 1.670 mg/kg in second harvest. P. ostreatus had a good potential for removing Fe, Mn, Zn and Cu from soils of metal scrap sites which contained these heavy metals in high concentrations above the WHO and USEPA standards. Pleurotus ostreatus can be used to amend heavy metal polluted sites.
Keywords: environmental pollution, mycoremediation, metal scrap site, Pleurotus oystreatus, heavy metals
Full Text:
INTRODUCTION
Contamination of soil environment by heavy metals is becoming prevalent across the globe (Abioye et el., 2010, Nilanjana et al., 2007). There is a steady increase in their concentration in all habitats owing to coal and metal ore mining, chemical manufacturing, petroleum mining and refining, electroplating, paints and dye, as well as battery making industries (Sobha t al., 2007, Gazso, 2001). Metal scrap is used to describe recyclable and other materials that are left over from the utilization of vehicles, building supplies, computers and other electronic gadgets. If heavy metals from such sites are not removed or degraded at once, they get immobilized on soil particles, leach into groundwater, and accumulate in many interlinking food chains because of their persistent nature (Cossich et al., 2002; Klimmek et al., 2001; Adelekan and Abegunde, 2011). Minute quantities of Se, Fe, Zn, Mn and Cu are common in our environment and are essential for the human metabolism by serving as enzyme activators (Yamaca et al. 2007). However, high concentrations of these elements can cause acute or chronic toxicity (Turkekul et al. 2004; Yamaca¸ et al. 2007). Heavy metal toxicity can result in damaged or reduced mental and central nervous function, lower energy levels, and damage to blood composition, lungs, kidneys, liver, and other vital organs (Ruiz-Manriques et al., 1998). Long-term exposure may result in slowly progressing physical, muscular, and neurological degenerative processes that mimic Alzheimer's disease, Parkinson's disease, Wilson’s disease muscular dystrophy, and multiple sclerosis. Repeated long-term contact with some metals or their compounds may even cause cancer (Ruiz-Manriques et al., 1998, International Occupational Safety and Health Information Centre, 1999). Fungus belongs to groups of organisms with very well known heavy metal sorption capacity and excellent metal uptake (Purvis, 1996). Mushrooms can build up large concentrations of some heavy metals, particularly cadmium (Cd), mercury (Hg), lead (Pb) and Cu (Kalac and Svoboda 2004, Kalac 2009). Many studies (Kalac and Svoboda 2005, Kalac et al. 1991) revealed a high ability of mushrooms to accumulate common pollutants present in the biosphere, mainly heavy metals and radionuclides. Various quantities of heavy metals have been observed in mushroom fruiting bodies of different mushrooms collected adjacent to heavy metal smelters, landfills of sewage sludge, emission area (Courtecuisse, 1999; Svoboda et al. 2006; Antonijevic and Maric, 2008; Svoboda and Kalac 2003). Mycoremediation involves the use of fungi to degrade or sequester contaminants in the environment (Stamets, 1999); it relies on microbial enzymatic activities to remove the contaminants from the environment (Philip et al., 2005). Mycoremediation offers an ecofriendly and low-cost bioremediation technique because it is a natural process and does not usually produce toxic by-products. It also provides a permanent solution as a result of complete mineralization of the contaminants in the environment (Perelo, 2010). Arica et al, (2003) reported, the use of turkey tail mushroom and phoenix oyster mushroom mycelia to eliminate 97% mercury ion from water. As observed by Humer et al, (2004), mushroom degraded copper and chromium in treated woods. This research was carried out to determine the effectiveness of using Pleurotus ostreatus in the mycoremediation of heavy metals by evaluating the concentration of heavy metals in Pleurotus ostreatus cultivated in soil samples from the metal scrap sites.
MATERIALS AND METHODS
Soil sampling and experimental design:
Three metal scrap sites (Polyafran-1, Nyonni-2 and Industries-3) in Tamale established in 1999, 2005 and 2007 respectively were randomly selected for investigation. Soil samples were obtained from each site at depths of 0-5 cm (top soil) and 15-20 cm (sub soil) and immediately placed in a fresh tightly sealed sack bag. The soil was spread on a clean rubber sheet placed on a flat surface and air-dried in open under room conditions for 24 hrs. Afterwards, 5 g of sample was taken from the sieved soil (2 mm sieve) and put in polyethylene bags for further analysis. It was an experiment with four replicates and seven (7) treatments. The treatments used were site 1 top soil (S1T),site 1 sub soil (S1S), site 2 top soil (S2S), site 2 sub soil (S2S),site 3 top soil (S3T), site 3 sub soil (S3S), non-metal scrap site (FS). Soil samples from a non-metal scrap site, U.D.S (Nyanpkala), were used as a control.
Cultivation of mushroom:
Sawdust (98%) was mixed with lime (1%) and urea (1%) to decompose. The substrate was decomposed for a month with constant stirring (using the flat bladed shovel) to enhance uniform distribution of oxygen, nutrients and ensure constant temperature. After decomposition, the substrate was combined with the various soil samples in a white perforated polyethylene bag. The bags were filled with 10kg of the substrate both at the base and at the top of the bag. The various soil samples (5kg) were placed it the middle making each bag 25kg. A polyvinyl chloride (pvc) pipe was placed at the neck of the polyethylene bag and was covered with a cotton wool. The bags were steam pasteurized at a temperature of 115 °C to kill potential competitive microorganisms in a sterilizing tank after heating for 1-2 hours. After the substrate has cooled, the spawn of Pleourotus ostreatus (obtained from the Food Research Institute, Ghana) was then broadcast over the surfaces of the bags. This was done under sterile condition in a room without air movement (closed doors and windows). In 30days, the mycelium had grown from the spawn and had permeated into the substrate. The mushroom began to form around the edges of the bag perforations. The bags were maintained under optimal growth conditions of temperature (15 °C) and pH of 7.0. The humidity was maintained at 70-80% by watering the bags regularly to favor fruiting. Afterwards they were then transferred to dark air- conditioned room for the development of the fruiting bodies under mushroom shelves. Following the mycelium growth, the PVC pipe was then plugged out, including the paper and the cotton, to allow primordial formation to occur under suitable environmental conditions. Fruiting bodies stated developing and were harvested one week after transferring to the production room.
Harvesting and analysis of soils and mushroom tissues:
For the analysis of heavy metals, 0.5 g of the soil sample was weighed into a round bottom flask which was connected to a partial condenser and a water cooler. Concentrated H2SO4 (2.5 ml) and HNO3 (2.5 ml) was added to the soil in the round bottom flask. It was then heated for 30 minutes and allowed to cool to room temperature. After cooling, another 10 ml of HNO3 was added and heated for an hour. Distilled water (2 ml) was added after cooling to room temperature. Afterwards, it was heated again for 15 minutes and allowed to cool to room temperature again. The cooled water was then filtered and used for the test. Determination of the heavy metals (Fe, Cu, Mn, and Zn) concentration was done with an atomic absorption spectrophotometer (AAS model 210 VGP). The first and second harvests were made in the first and second weeks. The fruiting bodies were harvested by gently turning them out of the soil and analysed for Mn, Fe, Zn and Cu. The mushroom was dried at a temperature of 70 °C for 15 hours. Dried mushroom samples (0.5 g) from each treatment were digested using concentrated HNO3 (2.5 ml) and H2SO4 (2.5 ml) in a whole glass system which consisted of a round bottom flask, partial condenser and water cooler. Final measurements of heavy metal content of mushroom and soil sample were performed using the Atomic Absorption Spectrophotometer (AAS model 210 VGP)
RESULTS
Heavy metal analysis - soil from metal scrap sites:
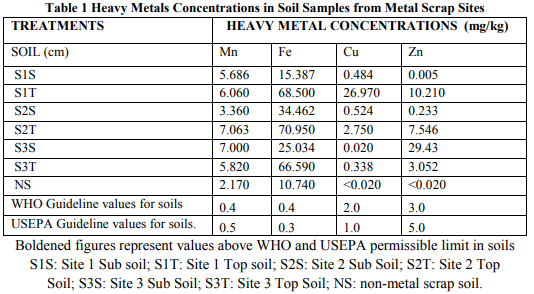
Soil analysis (Table 1) showed that Mn, Fe, Cu and Zn levels were higher in the top soils of Polyafran, and Nyonni stes. With the third site (Industries), sub soil concentrations of these metals were higher than the top soil. Heavy metals in top soils ranged from 0.338 mg/kg for Cu - 70.950 mg/kg for Fe with the sub soils ranging between 0.005 (Zn) – 34.46 mg/kg (Fe). Fe recorded the highest concentration in all soil samples followed Mn, Zn and Cu. Mn concentration was highest in site two top soils (S2T) with 7.063 mg/kg and least in site two sub soil (S2S) with 3.360 mg/kg. Fe was highest in site two top soils (S2T) with 70.95 mg/kg and least in site one sub soil (S1S) with 15.387 mg/kg. Cu was high in site one top soil (S1T) with 26.97 mg/kg and least in site three sub soil (S3S) with 0.02 mg/kg and lastly with Zn, it was high in site three top soils (S3T) with 29.43 mg/kg. The least concentrations of Mn, Fe, Cu and Zn were recorded in the control (non-metal scrap site).
Heavy metals concentrations in Pleurotus ostreatus:
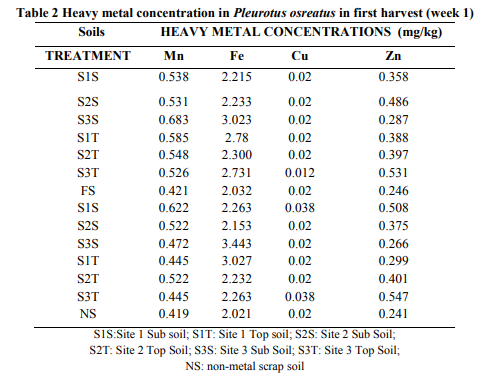
In the first harvest, (table 2), Fe content in P. ostreatus was the highest (2.215- 3.445 mg/kg). Mn was the next highly absorbed ranging from 0.455-0.688 mg/kg and it was followed by Zn (0.266-0.547 mg/kg). Cu was the least absorbed with a range of 0.012- 0.038 mg/kg. The highest concentration of Mn recorded was from site 2 subsoil (S2T) and the least recorded in site 1 topsoil (S1T). With Fe, the highest absorption was in site 3 subsoil (S3S) and the least absorbed from site 2 subsoil (S2S). The highest Cu observed was from site 1 subsoil (S1S) and the least observed from site 3 topsoil (S3T). Zn concentration was least in site 3 subsoil (S3S) and highest in site 3 topsoil (S3T).
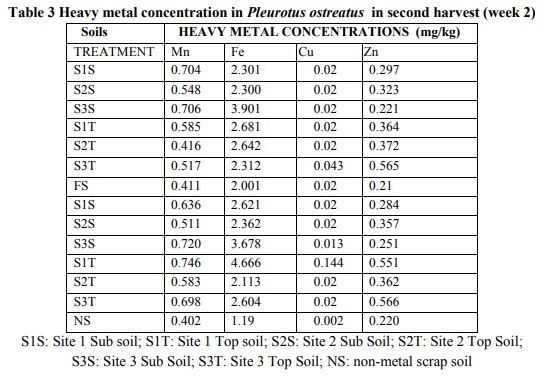
The highest absorption of the heavy metals by the Pleurotus ostreatus was in Fe, with Mn being the next highly absorbed (table 3). This was followed by Zn and the least was Cu. The lowest Fe concentration was 2.113 mg/kg from site 2 top soil (S2T) but was highly absorbed from site 1 topsoil (S1T) by 4.666 mg/kg. For Mn, the lowest absorption was 0.416 mg/kg from site 2 top soils (S2T) and the highest was 0.746 mg/kg. In Zn, it was least absorbed from site 3 subsoil (S3S) with 0.221 mg/kg and highly absorbed 0.566 mg/kg from site 3 topsoil (S3T). Again, for Cu, the least absorbed was 0.013 mg/kg from site 3 subsoil (S3S) and highly absorbed 0.144 mg/kg from site 1 topsoil (S1T).
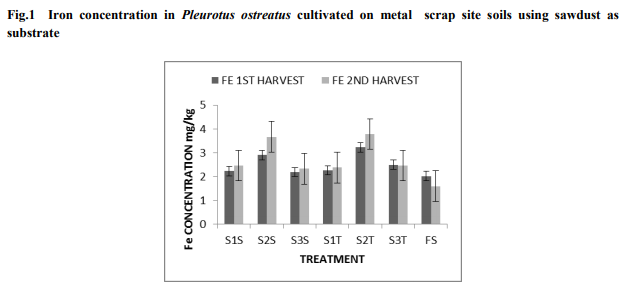
It was observed from both harvests that, there was a slight increase in heavy metal concentrations in the second harvest as compared to the first harvest. From tables 2 and 3, non-metal scrap soil (NS), showed the lowest absorption of Mn, Fe, Cu and Zn in Pleurotus osteatus for both harvest. From the first harvest, it was observed that, accumulation of the metals followed in a decreasing order of Fe > Mn > Zn > Cu. The same decreasing trend of Fe > Mn > Zn > Cu was observed in the second harvest Copper concentration in Pleurotus ostreatus: The mushrooms were able to absorb more of the Cu in the second harvest (grand mean- 0.287 mg/kg) than in the first harvest (grand mean0.022 mg/kg). The highest Cu concentration in the first harvest was 0.029 mg/kg from SIS and the least was 0.020 mg/kg in the rest of the treatments. The highest for the second harvest also recorded 0.082 mg/kg from S2S and the least being 0.0165 mg/kg from S2T. However there was no significant difference (P>0.05) between the treatments in both harvest. Manganese concentration in Pleurotus ostreatus: There was no significant difference (p>0.05) in Mn concentration in P. ostreatus between the treatments in both harvests .The second harvest recorded the highest concentration of Mn with a grand mean of 0.587 mg/kg as compared to the first harvest which had a grand mean of 0.520 mg/kg in the fruiting body of the P. ostreatus. In both harvest, the highest absorption of Mn occurred in S1S, with 0.580 mg/kg for the first harvest (table 2) and 0.670 mg/kg for the second harvest (table 3). S3T recorded the least of Mn for the first harvest with 0.486 mg/kg and S1T recorded the least of Mn for the second harvest with 0.499 mg/kg. Non-metal scrap soil (NS) recorded the least as compared to the other treatments (scrap soils) with 0.420 and 0.407 mg/kg for first and second harvest respectively. Zinc concentration in Pleurotus ostreatus: Zinc concentration varied significantly (p0.05) in the Fe concentration between the treatments in the second harvest but the Fe concentration was generally higher as compared to the first harvest (fig.1). However, there was a significant difference (p<0.05) between the treatments for the first harvest. In the first harvest, Fe recorded the highest concentrations in S2S and S2T with 2.904 and 3.233 mg/kg respectively. The least concentration recorded was 2.193 mg/kg for S3S (table 1). During the second harvest, the highest concentration of Fe was in S2T with 3.79 mg/kg and the least recorded 2.33 mg/kg from S3S (table 2). NS absorbed the least concentration as compared with the other treatments (scrap soils) with concentrations of 2.027 and 1.60 mg/kg for first and second harvest respectively.
DISCUSSION
Heavy metal concentrations in metal scrap soils: S1T and S2T have been in business not for long (7 and 5 years) and hence, the accumulation had been more at the top soils. Large quantities of heavy metals are introduced into the environment or ecosystems as a result of urbanization and industrial process over a period of time and this causes the heavy metals to gradually move from the top to the sub soil (Begume et al., 2009). High concentrations of Zn were recorded at S1T, S2T and S3S and these exceeded WHO and USEPA permissible limits. High Zn concentrations recorded for S3S may be due to the fact that site three has been used for scrap for a longer period (12 years) and hence, the Zn metal may have leached into the sub soils. High levels of Zn in the soil are associated with activities such as smelting which pollutes the soil (Samuel et al., 2010). Fe levels exceeded WHO and USEPA standards in all the soils and this may be attributed to the corrosive nature of Fe which increases its absorption in the soil.
Heavy Metals content in Pleurotus ostreatus:
Fungi and algae are potential biosorbents for heavy metals (Volesky and Holan, 1995). Cell walls are composed of structural polysaccharides, proteins and lipids that offer metal-binding functional groups (Veglio and Beolchini, 1997). Mushroom absorb and mineralize heavy metals in the soil samples by extracellular digestion of these heavy metals (Hitivani and Mecs, 2003; Stamets, 2005). Fe was the most accumulated by P. ostreatus (3.233 and 3.79 mg/kg d.w.) both in the first and second harvests respectively of all the soils of metal scrap sites. This is an indication of the ability of mushroom to absorb and the scavenging of metals from polluted sites (Malik, 2004) which are due to purifying abilities of mushrooms (Oudot, 1990; Barr and Aust, 1994; Stamets, 2005). Fe was highly absorbed in the second harvest than the first harvest. This can be attributed to the time interval between the harvests because as mushroom grows with time, it breaks down and absorbs or mineralizes environmental pollutants into non-toxic form (Hamman, 2004). Mushrooms of the second harvest had more time to grow, to penetrate and to absorb the Fe. Fe is the most common element (by mass) forming the planet earth as a whole (Samuel et al., 2010). Originally, Fe recorded the highest levels in all the soil samples and this may have accounted for its elevated accumulation in P. ostreatus the higher the initial concentration of metals, the higher their uptake by the mushroom. Levels of Mn were higher compared to WHO and the USEPA permissible guidelines (tables 1, 2 and 3); Mn was the second highly absorbed metal in P. ostreatus probably due to its nature of being the most abundant metals in soil (Samuel et. al., 2010). Higher levels of Zn in the first harvest as compared to the second harvest with the exception of S2S and S3T may be due to the high levels of the Zn in the soil since Zn is an element of moderate abundance in the earth crust (Samuel et al., 2010). This suggests that the mushroom absorbed the heavy metals relative to their initial concentrations in the soil. The non-metal scrap soil (NS) absorbed the lowest, indicating that, the mushroom is able to absorb higher levels of heavy metals from heavy metal contaminated soils. Copper was the least accumulated in the fruiting body P. ostreatus, due to its low levels in the soils: The highest amount absorbed was 0.0820 mg/kg and the least was 0.00165 mg/kg in both harvests (table 1); Cu concentrations are usually low, except areas near copper-related industries (Kies, 1989).
CONCLUSION
(Mn, Cu, Fe and Zn contents were detected the fruiting bodies of the P. ostreatus; there was a differences in Fe and Zn accumulation throughout the treatments were statistically significant (p Mg > Zn > Cu. The uptake of Cu was considerably lower in all the treatments and it was the same treatment that recorded the least from the metal scrap soil. Pleurotus ostreatus offered a possibility of amending heavy metal contaminated soils from scrap sites.
References:
1. Abioye, O. P., Abdul Aziz, A., Agamuthu, P. (2010). Enhanced Biodegradation of Used Engine Oil in Soil Amended with Organic Wastes. Water Air and Soil Pollution. 209: 173 – 179.
2. Adelekan, B. A. and Abegunde, K. D. (2011). Heavy metals contamination of soil and groundwater at automobile mechanic villages in Ibadan, Nigeria International Journal of the Physical Sciences Vol. 6 (5), pp. 1045-1058.
3. Antonijevic, M., Maric, M. (2008). Determination of the content of heavy metals in pyrite contaminated soil and plants. Sensors 8:5857–5865.
4. Arica, M.Y.; Arpa, C.; Kaya, B.; Bektas, S.; Denizilli, A. And Gene, O. (2003).Comparative biosorption of mercuricion from aquatic systems by immobilized live and heatinactivated Trametes versicolor and Pleurotus sajo-caju. Bioresource Technology 89(2):145-154.
5. Barr, D.P. and Aust, S.D. (1994). Mechanisms of white fungi use to degrade pollution. Crit. Rev. Environ. Sci. Technol. 28 (2): 79 – 87.
6. Begume, A., Ramaiah, M., Harikrishna, Khan I., Veena, K. (2009). Analysis of Heavy Metals Concentration in Soil and Litchens from Various Localities of Hosur Road, Bangalore, India. E-J. Chem., 6(1): 13-22. http://www.e-journals.net.
7. Cooper, D.C., Neal, A.L., Kukkadapu, R.K., Brewe, D., Coby A, Picardal F.W. (2005). Effects of Sediment Iron Mineral Composition on Microbially Mediated Changes in Divalent Metal Speciation: Importance of Ferrihydrite. Geochim. Cosmochim. Acta, 69: 1739-1754.
8. Cossich, E S., Tavares, C.R.G., Ravagnani, T.M.K. (2002). Biosorption of chromium (III) by Sargassum sp biomass. August 15 (Cited 26 October, 2005). Available from http//www.ejbiotechnology.info/content/vol 5/issue15/full15/index html. ISSN 0717- 3458. Elec. J. Biotech., 5(2): 133-140.
9. Courtecuisse, R. (1999). Collins guide to the mushrooms of Britain and Europe. Harper Collins Publishers, London.
10. Gazso, L.G. (2001). The Key Microbial Processes in the Removal of Toxic Metals and Radio nuclides from the Environment. A review. Cent. Eur. J. Occup. Environ. Med., 7(3): 178–185.
11. Hamilton-Taylor J., Smith, EJ, Davison, W., Sugiyama, M. (2005). Resolving and Modeling the Effects of Fe and Mn Redox Cycling on Trace Metal Behavior in a Seasonally Anoxic Lake. Geochim. Cosmochim. Acta. 69: 1947-1960.
12. Hamman, S. (2004). Bioremediation capability of white rot fungi. B- 1570, Review article, spring 2004.
13. Hitivani, N. and Mecs, L. (2003). Effects of certain heavy metals, on the growth, dye decolouration and enzyme activity of Lentinula edodes Ectoxicology and Environmental safety 55(2):199-203. http://www.microrestoration-info.com (Assesed 12-11-2012).
14. Humer, M.; Bokan, M; Amartey, S.A.; Sentijure, M. Kalan, P. and Pohleven, F. (2004). Fungal bioremediation of copper, chromium and boron treated wood as studied by electron pragmatic resonance. International Biodeterioration and Biodegradation 53:25-32.
15. International Occupational Safety and Health Information Centre 1999. Metals in Basics of Chemical Safety, Chapter 7, Sep. Geneva: International Labour Organization.
16. Kalac, P. (2009) Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chem 113:9–16.
17. Kalac, P., Burda, J., Staskova, I. (1991) Concentration of lead, cadmium, mercury and copper in mushroom in the vicinity of a lead.
18. Kalac, P., Svoboda, L. (2005) A review of trace element concentrations in edible mushrooms. Food Chem 69:273–281.
19. Kies, C. (1989). Copper bioavailability and metabolism. 1st ed. Plenum Press. New York.
20. Klimmek, S., Stan, H.J., Wilke, A., Bunke, G., Buchholz, R. (2001). Comparative analysis of the biosorption of cadmium, lead, nickel and zinc by Algae. Environ. Sci. Technol., 35: 4283-4288.
21. Malik, A. (2004). Metal bioremediation through growing cells. Envi. Interna. 30: 261-278.
22. Nilanjana, Das; R. Vimala, and P. Karthika, (2006, 2007). School of biotechnology, chemical and biomedical engineering, VIT University, Vellore 632014, India.
23. Oudot, J. (1990). Selective migration of low and medium molecular weight hydrocarbon in petroleum contaminated terrestrial environment. Oil and chemical pollution 6:251-261.
24. Perelo, L. W. (2010) Review: In situ and bioremediation of organic pollutants in aquatic sediments. Journal of Hazardous Materials 177: 81 – 89.
25. Philip, J. C., Atlas, R. M. (2005). Bioremediation of contaminated soil and aquifers. In: Bioremediation: Applied Microbial Solution for Real- World Environmental Clean Up. Atlas, R. M., and Jim, C. P. (ed.) ASM Press, ISBN 1-55581- 239-2, Washington, D.C., pp.139.
26. Ruiz-Manriquez A., Magaña, P. I., Lopez, R., and Guzman, R. (1998). Biosorption of Cu by Thiobacillum ferrooxidans, Bioprocess Engineering 18, pp. 113-118.
27. Samuel, O., Florence, E., Emmanuel, A. and Fredrick, A., (2010). Human risk assessment and epidemiological studies from exposure to toxic chemicals in Tarkwa-Nsuaem municipality, Prestia Huni Valley District and Cape Coast Metropolis Ghana, pp 46-49.
28. Sobha, K., Poornima, A1., Harini, P1., Veeraiah (2007). k2 a study on biochemical changes in the fresh water fish, catla catla (hamilton) exposed to the heavy metal toxicant cadmium chloride kathmandu university journal of science, engineering and technology vol.i, no.iv.
29. Stamets, P. (1999). Undated. "Helping the Ecosystem through Mushroom Cultivation." Adapted from Stamets, P. 1998. "Earth's Natural Internet." Whole Earth Magazine, Fall.
30. Stamets, P. (2005). Mycelium Running. How mushroom can help save the world Ten speed Press, Berkeley/Toronto. 1st Edition. 339 pp.
31. Turkekul, I., Elmastas, M. and Tuzen, M., (2004) Determination of iron, manganese, zinc, lead and cadmium in mushroom samples from Tokat, Turkey, Food chem. 84, 389-392.
32. Veglio, F., Beolchini, F. (1997): Removal of metals by biosorption: a review. Hydrometallurgy 44, 301-316.
33. Volesky, B., Holan, Z.R. (1995): Biosorption of heavy metals. Biotechnology Programme. 11, 235-250
34. Yamaca, M., Yldz, D., Sarku¨rkcu¨, C. C., Elikkollu, M., Halil, Solak M. (2007) Heavy metals in some edible mushrooms from the Central Anatolia, Turkey. Food Chem 103(2):263–267.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License