IJCRR - 5(11), June, 2013
Pages: 71-79
Date of Publication: 18-Jun-2013
Print Article
Download XML Download PDF
IN VITRO ASSESSMENT OF THE ANTIMICROBIAL EFFECT OF ETHIOPIAN MULTI-FLORA HONEY ON METHICILLIN RESISTANT STAPHYLOCOCCUS AUREUS
Author: Alem Getaneh, Yeshambel Belyhun, Feleke Moges, Belay Anagaw, Bikes Destaw, Chandrashekhar Unakal, Andargachew Mulu
Category: Healthcare
Abstract:Background: Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most significant human pathogens that cause both nosocomial and community-acquired infections worldwide which are associated with high morbidity and mortality rates with rapid development of resistance. Honey is one of the oldest traditional medicines considered to be important in the treatment of several human ailments including infections not responding to standard antiseptic and antibiotic therapy. Assessing the antimicrobial effect of honey on antibiotic resistant bacteria has a paramount importance. Objective: To assess in vitro antimicrobial effect of Ethiopian multi-flora honey against MRSA. Methods: The antimicrobial effect of Ethiopian multi-flora honey against MRSA was investigated by a tube dilution method for determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). A MRSA isolate was inoculated on nutrient broth medium containing various concentrations of honey solutions and incubated aerobically at 36-37oC. After overnight incubation the tubes were read macroscopically to confer the growth by comparing with that of the control tubes and were sub cultured on agar plate to determine the lowest concentration of the test honey that prevent any visible growth represents the MIC. The MBC could also be determined at the lowest concentration of honey that can kill 99.9% of the bacteria. Result: The mean MICs and MBCs were 11.25 % (v/v) and 16.25 % (v/v) for the \"Tazma mar\" from low land , 9.38 and 14.38 % (v/v) \"Tazma mar\" from high land , 23.12 and 28.12 % (v/v) red honey from low land, 33.12 and 38.12 % (v/v) red honey from high land, and 17.5 and 22.5% (v/v) white honey from high land areas respectively against MRSA. Conclusion: Honey has both a bacteriostatic and bactericidal activity. However, the \"Tazma mar\" honey antibacterial potency on both the test (MRSA) and control bacterial isolates was highly effective than other honey types.
Keywords: MRSA, Multi-flora Honey, MIC, MBC.
Full Text:
INTRODUCTION
The development of resistance to multiple antibiotics and increased disease transmission by bacteria in hospitals/communities has been recognized as the major challenges as the bacterial population that expresses the resistance phenotypes1,2 . MRSA is any strain of Staphylococcus aureus that has developed resistance to beta-lactam antibiotics3 , thus limiting the treatment options to very few agents such as vancomycin and teicoplanin4 . It is one of the most significant human pathogens that cause both nosocomial and community-acquired infections worldwide which are associated with high morbidity and mortality rates with rapid development of resistance.4, 5, 6 In health-care settings, MRSA is a frequent cause of surgical wound infections, urinary tract infections, bloodstream infections (sepsis), and pneumonia7 . Antibiotic use is generally considered the main risk factor for antibiotic resistance. One of the possibilities to reduce the use of antimicrobial agents is the use of non-antibiotic compounds such as honey for the treatment of infections8 . The use of traditional medicine to treat infection has been practiced since the origin of mankind and honey produced by Apis mellifera or Apis mellipodae is one of the oldest traditional medicines considered to be important in the treatment of several human ailments9 , as well as the micro flora isolated from Ethiopian honey demonstrated bacteriocin production potentially active against Staphylococcus aureus10 . Honey is an ancient remedy for the treatment of infected burns and wounds6, 11 which has recently been rediscovered’ by the medical profession, particularly where conventional or standard modern therapeutic agents failed to cure infections11, 12 . World Health Organization (WHO) estimated that 80% of the world population depends on traditional medicine to treat them13, 14, 15. A similar proportion of the population living in developing countries including Ethiopia rely on herbal medicine on harvested wild plants for their primary health care14, 15, 16 . Honey is the natural sweet substance which is widely used in traditional medicine throughout the world14, 17. It has been successfully used on infections not responding to standard antiseptic and antibiotic therapy and that the full potential of honey has been recognized12. Since ancient times, honey has been known to possess antimicrobial properties which rapidly clears infection and protects wounds from becoming infected, as well as wound-healing activity. Indeed, the in vitro activity of honey against antibiotic-resistant bacteria and the reported successful application of honey in the treatment of chronic wound infections that were not responding to antibiotic therapy have attracted considerable attention18, 19 . However, information regarding on the effectiveness of honey against MRSA is scarce particularly in Sub Saharan Africa including Ethiopia. The present study is therefore, aimed at to assess the in vitro antimicrobial effects of Ethiopian multi-flora honey against MRSA isolated from clinical specimens.
METHODS AND MATERIALS
Study design and period
An experimental study was conducted to assess the antimicrobial effect of Ethiopian multi-flora honey against MRSA at University of Gondar Teaching Hospital from February 2012 to May 2012. Study Area The study was conducted at University of Gondar Teaching Hospital, Gondar. As a teaching hospital and research center, it is also a center that supplies highly competent health professionals and scientists to the nation20 . Sample source and preparation Source and preparation of honey samples Six honey samples viz., white honey, red honey and “tazma mar” each from highland and lowland areas, produced by honeybee (Apis mellifera) and sting-less bee (Apis mellipodae) were evaluated. Honey samples were obtained from local market (farmer) and Amar Associational Organization which distributes refined honey to the local markets of Amhara found in Amhara region of North West Ethiopia, Gondar, where different kinds of flowering plants grow naturally were used. These honey samples were collected in screwed bottles and kept in a cool and dry place (at room temperature). Before the experiment each honey samples were filtered using a sieve to remove debris of honey. Different concentrations of honey solutions were prepared in sterile distilled water and then filtered in a micro porous filter membrane (diameter=47mm, pore size=0.45µm), which is capable of preventing the passage of bacteria and spores. The concentration used for this test was 10%, 15%, 20%, 25%, 30%, 35%, 40% and 45% for white and red honey, 5%, 10%, 15%, 20%, 25%, 30%, 35%, and 40% for “tazma mar”. Source and preparation of test organism (MRSA) and control organisms Four isolates of preserved MRSA were evaluated. Three reference organisms: Staphylococcus aureus (ATCC-25923), Pseudomonas aeruginosa (ATCC-27853) and Escherichia coli (ATCC25922) were included as a control. All these test and control organisms were obtained from the Department of Microbiology, University of Gondar, North West Ethiopia. Suspension of the organism was prepared with normal saline and the inoculum density was adjusted with turbidity of 0.5 McFarland standards21 . General principle of the test Antimicrobial susceptibility test measures the ability of an antimicrobial agent to inhibit bacterial growth in vitro. This ability may be estimated by macro broth or tube dilution, micro broth or well dilution, antimicrobial gradient method, agar dilution tests or disk diffusion tests (Bauer-Kirby Procedure) 21 .
Determination of MIC and MBC
The MICs and MBCs of the six honey samples for MRSA isolates and reference organisms were conventionally determined twice for each isolate by the tube dilution method then sub cultured on agar plate. This technique was done by mixing Nutrient broth with a known volume (2ml) of diluted and filtered honey solution: 5% V/V, 10% V/V, 15% V/V, 20% V/V, 25% V/V, 30% V/V, 35% V/V, 40% V/V and 45% V/V per 10 ml of media were used. A known volume of honey (2ml) was added with the prepared Nutrient broth to get a final volume of 10 ml and then a loop full of standardized bacterial suspension of approximately 104 CFU/ml (2 micro liter/0.002 ml) was inoculated in each tube. After overnight incubation aerobically at 36-37oC the tubes were examined macroscopically for visible evidence of bacterial growth in the form of turbidity by comparing with the control tubes. Two control tubes were employed; one was a row of positive control tubes containing only the broth/growth medium and each of the microorganisms, while the other was a negative control which consisted of a row of tubes containing different concentrations of honey with no organism. After which they were read macroscopically to confer the growth were sub cultured. The last tube which showed visible growth and all the tubes in which there was no growth in nutrient broth or from tube dilution step were sub cultured on dried MSA and MHA media to determine the lowest concentration of the test honey that prevent any visible growth. So the lowest concentration of honey that completely inhibits or prevents visible growth represents the MIC. The MBC was therefore determined at the lowest concentration of honey that can kill 99.9% of the bacteria or that produce a sterile culture.
Pretest and data quality control
Pretest was conducted at University of Gondar teaching hospital laboratory. The method was tested on MRSA and quality control organisms: S. aureus (ATCC-25923), P. aeruginosa (ATCC27853) and E. coli (ATCC-25922). Sensitivity test was done against honey of different concentration and methicillin (for MRSA) for its reliability and validity before it was used for actual experiment. To increase the data quality the experiment was also be under advisors supervision and use of proper sterilization techniques.
Data processing and analyses
The collected and cleared data was analyzed using descriptive statistics. Results were displayed using tables and also expressed in words.
Ethical consideration
The study was conducted after obtaining institutional ethical clearance from the ethical review committee of School of Biomedical and Laboratory Sciences, Collage of Medicine and Health Sciences, University of Gondar.
RESULTS
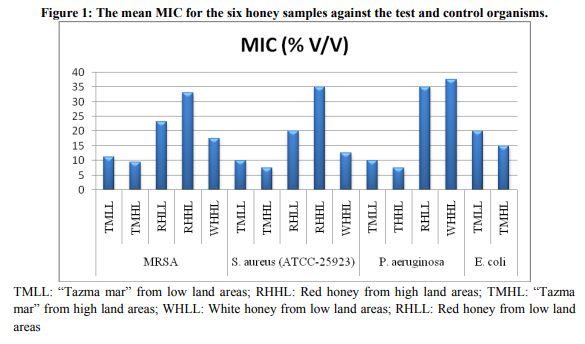
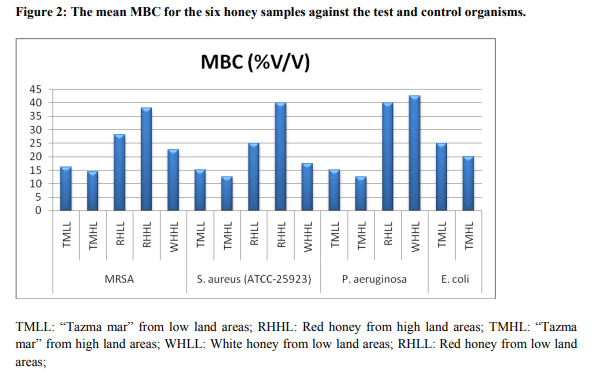
The mean MICs and MBCs of the six honey samples with clinical isolates of MRSA and control organisms: S. aureus (ATCC-25923), P. aeruginosa (ATCC-27853) and E. coli (ATCC25922) are presented on Table 1. The MIC and MBC results are also illustrated in figure 1 and 2 below. White honey from low land areas showed no antibacterial activity against all the test and control organisms. The mean MIC and MBC of “tazma mar” for all MRSA isolates ranged from 7.5 to 15% (v/v) and 12.5 to 20% (v/v), respectively, and other honey types ranged from 17.5 to 35 and 22.5 to 40% (v/v), respectively. The mean MIC and MBC value of all the four test organisms (MRSA) were 11.25% (v/v) and 16.25% (v/v) for the “tazma mar” honey from low land areas and 9.38% (v/v) and 14.38 % (v/v) for high land areas. The result also revealed that the mean MIC and MBC value for the red honey from low land areas were 23.12 % (v/v) and 28.12 % (v/v) and for high land areas were 33.12 % (v/v) and 38.12 % (v/v); whereas the white honey from high land areas was 17.5 % (v/v) and 22.5% (v/v), respectively. The mean MIC and MBC value of the control S. aureus (ATCC-25923) were 10% (v/v) and 15% (v/v) for the “tazma mar” honey from low land areas; whereas, 7.5% (v/v) and 12.5 % (v/v) for the “tazma mar” honey from high land areas, respectively. The result also showed that the mean MIC and MBC value of the red honey from low land areas were 20 % (v/v) and 25 % (v/v), and from high land areas were 35% (v/v) and 40 % (v/v); whereas 12.5% (v/v) and 17.5% (v/v) for the white honey from high land areas, respectively. The MIC and MBC of P. aeruginosa (ATCC-27853) for “tazma mar” honey from low land areas was 10% and 15% (v/v); whereas high land areas was 7.5% and 12.5% (v/v), respectively. The red honey from the low land areas show the mean MIC and MBC value of 35% (v/v) and 40% (v/v), and white honey from high land areas show 37.5% (v/v) and 42.5% (v/v), respectively. Red honey from high land areas and white honey from low land areas showed no activity against P. aeruginosa (ATCC-27853). The MIC and MBC of E. coli (ATCC-25922) for “tazma mar” from low land areas was 20% and 25% respectively; whereas high land areas was 15% and 20%, respectively. Red and white honey showed no activity against E. coli.
DISCUSSION
Honey one of the oldest traditional medicines, is being used effectively as a dressing for wounds, burns and skin ulcers22. It completely inhibits the growth of gram negative and gram positive bacteria11, 13 . Honey has previously been shown to have wound healing and antimicrobial properties, but this is dependent on the type of honey, geographical location and flower from which the final product is derived24. The present study tested the antimicrobial activity of six honey samples against MRSA isolates and control organisms: S. aureus (ATCC-25923), P. aeruginosa (ATCC-27853) and E. coli (ATCC25922). Even if honey has an antimicrobial property against MRSA, many studies have demonstrated that not all honey samples have the same degree of antibacterial activity. Therefore, the sensitivity of MRSA isolates and control organisms cannot be compared using the results from different studies, as the honeys used in the studies may have had widely differing antimicrobial activity. The MIC and MBC value determined with the MRSA and control S. aureus (ATCC-25923) strains in this study indicate that there is no much difference in sensitivity (effectiveness of honey to inhibit growth or to kill the bacteria) to honey. Hence, honey has potential in the decontamination of wounds colonized by antibiotic resistant strains of bacteria and nonresistant strains. In the present study the mean MIC value of “tazma mar” honey from low land and high land areas against MRSA (11.25 and 9.38 % v/v) were close to those previously determined with an agar well diffusion assay in Ireland that showed a lower MIC was observed (12.5% v/v) for MRSA isolates. For the E. coli and Pseudomonas strains equivalent MICs were observed (12.5% v/v) 24, which are lower than the MIC value of the red and white honey in the present study. In this study the MIC value of “tazma mar” [11.25% (v/v)] which was almost in line with MIC values with the study conducted in Addis Ababa reported that “tazma mar” honey was found to be effective against low concentration (10%) for S. aureus 25 . In contrast, another study12 reported that the MIC value of honey, was 4% (v/v) for S. aureus (ATCC 25923), and 8% (v/v) for P. aeruginosa (ATCC 27853) and study conducted in New Zealand showed the mean MIC value of two honeys against MRSA (2.98 and 3.1% v/v) which has a lower MIC value with the present study. This difference might be due to the variation in antimicrobial activities of honey in different geographical locations and could be attributed to the materials available for honey bees in preparing their valuable honey. This supports the existing traditional practice of using honey to treat wound infections6, 11, 18, 19 . However, the mean MIC and MBC of red honey on MRSA in the present study was found to be higher [23.12% (v/v) and 28.12 % (v/v), and 17.5% and 22.5% for white honey] as compared with a report from Nigeria where the antimicrobial activity of honey was found to be (the MIC and MBC of the honey) effective on S. aureus at 4%, while on P. aeruginosa MIC at 5% and MBC at 6% using the agar diffusion method26. This variation in the antibacterial activity of honey might be due to the type of honey or the source of the nectars (the flowers from which bees gathered nectar to produce the honey), since flora source determines many of the attributes of honey11 and the methodology may have contributed to the difference in the antimicrobial activities of honey. Inoculum density may also contribute to the variation in the antimicrobial activity of honey. In the present study, the percentage by volume of red honey that completely prevents MRSA is ranged from 22.5-35%, P. aeruginosa 35%. A similar study conducted in Malaysia showed that honey was found to be effective against MRSA, inhibited from concentrations of 25-30% using an agar incorporation technique6 . The MIC values obtained in this study demonstrate that two honeys (“tazma mar” from both high land and low land areas) produced by stingless bee (Apis mellipodae) were more effective in inhibiting MRSA, S. aureus (ATCC25923) and gram negative bacteria (P. aeruginosa (ATCC-27853) and E. coli (ATCC25922) in in vitro tests than other honey types produced by honey bee (Apis mellifera). This may be due to the type of bees that produce honey. The sensitivity of strains of MRSA to honey including “tazma mar” was almost similar to that of the control S. aureus (ATCC-25923). Honey also has a range of viscosities; these can be altered depending on the temperature at which they are measured. The color and consistency of honey is not only affected by the source of flower from which the nectar was collected but is also affected by variables such as weather and climatic changes27. In this study, white honey obtained from low land areas had no any antibacterial activity against any of the test and control organisms at 45% (v/v) dilutions. This may be due to the type, geographical difference and low viscosity comparing to other honey types by visual inspection. “Tazma mar” honey showed antibacterial effect against E. coli (ATCC-25922), with the strongest activity seen against MRSA, S. aureus (ATCC25923) and P. aeruginosa (ATCC-27853). The antimicrobial effects of other honey samples were more with MRSA strains and control organism of S. aureus (ATCC-25923) than with the other bacteria tested: P. aeruginosa (ATCC-27853) and E. coli (ATCC-25922). E. coli (ATCC-25922) was not inhibited by 45% (v/v) honey (other than “tazma mar”) solution, which is the highest concentration achievable in this assay. The Staphylococci were more sensitive to the honey than the P. aeruginosa (ATCC-27853) and E. coli (ATCC-25922). The reason for this might be due to difference in cellular organization. The MIC and MBC of P. aeruginosa (ATCC-27853) for “tazma mar” from low land areas was 10% and 15% respectively; whereas from high land areas was 7.5% and 12.5% ,respectively which is similar result to that of Staphylococcus isolates. MRSA, S. aureus (ATCC-25923), and P. aeruginosa (ATCC-27853) were more susceptible for “tazma mar” than E. coli (ATCC25922). The acidic pH of honey (normally pH 3.2 – 4.5) also limits or inhibits the growth of many organisms. Animal bacterial pathogens generally need a pH of 7.2 – 7.4 for optimum growth28. In this study the pH range of the six types of honey was from 3.5–5.0. Moreover, among the six different types of honey, “tazma mar” honey was more acidic as compared to others.
CONCLUSION
Honey had antimicrobial properties against MRSA organisms tested. It has both a bacteriostatic and bactericidal activity when tested in vitro using dilution of honey. The antibacterial potency of “tazma mar” honey on both the test organism MRSA and control bacterial isolates was highly effective when compared to other honey types. The test organism, MRSA and Staphylococcus aureus (ATCC-25923) were found to be more susceptible than other control bacterial strains; Pseudomonas aeruginosa (ATCC-27853) and Escherichia coli (ATCC-25922).
ACKNOWLEDGEMENTS
The authors are indebted to the University of Gondar Teaching Hospital Laboratory and Department of Medical Microbiology for support and facilities during this the study. Also, authors acknowledge the great help received from the scholars whose articles cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. Authors are grateful to IJCRR editorial board members and IJCRR team of reviewers who have helped to bring quality to this manuscript.
COMPETING INTERESTS
We declare that there is no conflict of interest
References:
1. Chandrashekhar Unakal, Basappa Kaliwal. Phenotypic characterization and risk factors of nosocomial Staphylococcus aureus from Health Care Centers. Adv. in Microbiol., 2012; 2: 122-128
2. Amare Gebrehiwot, Wubishet Lakew, Feleke Moges, Belay Anagaw, Gizachew Yismaw, Chandrashekhar Unakal et al. Bacterial profile and drug susceptibility pattern of neonatal sepsis in Gondar University Hospital, Gondar Northwest Ethiopia. Der Pharmacia Lettre, 2012, 4 (6):1811-1816.
3. Francois P, Schrenzel J. "Rapid diagnosis and typing of Staphylococcus aureus". Staphylococcus: Molecular Genetics/ Book 2008; 2: 19.
4. Baddour MM, Abuelkheir MM, Fatani AJ. Trends in antibiotic susceptibility patterns and epidemiology of MRSA isolates from several hospitals in Riyadh, Saudi Arabia. Annals of Clinical Microbiology and Antimicrobials 2006; 5 (30): 1-11.
5. Shittu AO, Okon K, Adesida S, Oyedara O, Witte W, Strommenger B, Layer F, Nübel U. Antibiotic resistance and molecular epidemiology of Staphylococcus aureus in Nigeria. BMC Microbiology 2011; 11: 92.
6. AL-Haj NA, Amghalia E, Shamsudin MN, Abdullah R, Mohamed R, Sekaw Z. Antibacterial activity of honey against methicillin-resistant Staphylococcus aureus. Research Journal of Biological Sciences 2009; 4(8): 943-947.
7. Klatz R, Goldman R. Community associated MRSA: Anti-Aging Approaches for a Superbug Survival Strategy: Townsend Letter. Anti-Aging Medicine 2009. http://www.worldhealth.net/
8. Stobberingh EE. Antibacterial activity of honey against ESBL-producing strains. Antibacterial activity of L-Mesitran Ointment and L-Mesitran Soft 2010.
9. Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pacific Journal of Tropical Biomedicine 2011; 1691(11): 154-160.
10. Chandrashekhar Unakal, Gizachew Yismaw, Amare Gebrehiwot, Mengistu Endris and Feleke Moges. Effect of bacteriocin produced from Enterococcus faecium against drug resistant bacterial isolates. International Journal of Biomedical and Advance Research 2012; 3(12): 881-886
11. Molan PC. The antibacterial activity of honey. Bee World 1992; 73: 5-28.
12. George NM, Cutting KF. Antibacterial Honey: in vitro activity against clinical isolates of MRSA, VRE, and other multiresistant Gram-negative organisms including Pseudomonas aeruginosa. Wounds 2007; 19: 231-236.
13. Ashebir M, Ashenafi M. Assessment of the antibacterial activity of some traditional plants on some food-borne pathogens. Ethiopian Journal of Health Development 1999; 13(3): 211–216.
14. Chauhan A, Pandey V, Chacko KM, Khandal RK. Antibacterial activity of raw and processed honey. Electronic Journal of Biology 2010; 5(3): 58-66.
15. Dikasso D, Lemma H, Urga K, Ambaye C, Ayele A, Yersaw K. Investigation of the antibacterial effect of papaya (carica papaya) seeds on three pneumonia causing bacteria. Ethiopian Journal of Health Science 2002; 12(1): 47-53.
16. Abebe D. The development of drug research. EHNRI News Letter 1996; 1: 5-6.
17. Subrahmanyam M, Hemmady A, Pawar SG. Antibacterial activity of honey on bacteria isolated from wounds. Annals of Burns and Fire Disasters 2001; 14 (1): 1-5.
18. Osman OF, Mansour IS, EI-Hakim S. Honey compound for wound care: A preliminary report. Annals of Burns and Fire Disasters 2003; 16(3): 1-7.
19. Kwakman PHS, Van den Akker JPC, Guclu A, Aslami H, Binnekade JM, Boer L, Boszhard L, Paulus F, Middelhoek P, Velde AA, Vandenbroucke-Grauls CM, Schultz MJ, Zaat SAJ. Medical-grade honey kills antibiotic-resistant bacteria in Vitro and eradicates skin colonization. Clinical Infectious Diseases 2008; 46: 1677–82.
20. Five Years Strategic Plan (2010/11 to 2014/15). Gondar University teaching Hospital, 2011.
21. Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: General principles and contemporary practices. Clinical Infectious Diseases 1998; 26: 973–80.
22. Mulu A, Diro E, Tekleselassie H, Belyhun Y, Anagaw B, Alemayehu M, Gelaw A, Biadglegne F, Desalegn K, Yifiru S, Tiruneh M, Kassu K, Nishikawa T, Isogai E. Effect of Ethiopian multiflora honey on fluconazole-resistant candida species isolated from the oral cavity of AIDS patients. International journal of STD and AIDS 2010; 21:1-5.
23. Mulu A, Tessema B, Derbie F. In vitro assessment of the antimicrobial potential of honey on common human pathogens. Ethiopian Journal of Health Development 2004; 18(2):107-111.
24. Sherlock O, Dolan A, Athman R, Power A, Gethin G, Cowman S, Humphreys H. Comparison of the antimicrobial activity of ulmo honey from Chile and manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complementary and Alternative Medicine 2010; 10: 47.
25. Mogessie A. The in vitro antibacterial activity of ‘Tazma mar’ honey produced by sting less bee. Ethiopian Journal of Health Development 1994; 8(2): 109–117.
26. Omoya, FO, Akharaiyi FC. A Pasture honey trial for antibacterial potency on some selected pathogenic bacteria. Journal of Natural Products 2009; 3(2010):05-11
27. Cooper RA, Molan PC, Harding KG. Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. Journal of Royal Society Medicine 1999; 92: 283-285.
28. Molan PC. Potential of honey in the treatment of wound and burns. American Journal of Clinical Dermatology 2001; 2(1): 13-9).

|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License