IJCRR - 5(11), June, 2013
Pages: 10-29
Date of Publication: 18-Jun-2013
Print Article
Download XML Download PDF
ANTIFUNGAL ANTIBIOTIC OF STREPTOMYCES SP. SS12: PRODUCTION AND CHARACTERIZATION
Author: Shipra Singh, Anita Rawat, Deepak Chand Sharma
Category: General Sciences
Abstract:Streptomyces sp. (strain SS12) was isolated from terrestrial soil of western U.P., India and found to exhibit antimicrobial activity against various pathogenic bacteria and fungi. Optimization of physical and chemical parameters for antibiotic production in submerged fermentation resulted in 1.75 fold increase in production level in terms of inhibition zone diameter. The bioactive compound was found thermo stable (121?C for 15min.), pH stable (from 4-9) and soluble in water and ethyl acetate. The antifungal antibiotic was found to have comparable activity with nystatin and could completely inhibit the spore germination and degradation of mycelia in test fungus (Aspergillus sp.). On supplementing 0.5% of the crude culture filtrate containing bioactive compound resulted in 76% inhibition of growth in submerged culture in compare to control.
Keywords: Antifungal antibiotic, Actinomycetes, Optimization, Streptomyces sp. SS12, Bioactive.
Full Text:
INTRODUCTION
Inappropriate and over prescription of antibiotics has resulted in the development of resistant microbial pathogens (WHO, 2012). The incidences of infections by opportunistic fungi are increasing at alarming rate, especially in patients with feeble immune systems. Many antifungal compounds exist but safe and effective antifungal drugs have not yet been developed because of the high degree of similarity between fungi and mammalian cells (Xu et. al., 2013). Therefore, amphotericin B, which was developed many years ago, is widely used for the treatment of deep-seated mycoses despite its serious side effects (Gallis et al., 1990). Azole group antifungal agents, miconazole, keto- conazole, fluconazole, and itraconazole are also being used clinically; but these medicines have nephrotoxicity and hepatotoxicity and cause vomiting and impotence (Fukai et al., 2003). This has consequently resulted in a strong demand for potent drugs that have least side effects and the search for the new antifungal metabolites remains as a challenging task. Actinomycetes are group of gram positive bacteria and the most prolific microorganisms for the production of antibiotics which are responsible for approximately two-thirds of the world’s naturally occurring antibiotics by the 1980s (Berdy, 1989). The antagonistic activity of actinomycetes to fungal pathogens is usually related to the production of antifungal compounds (Getha and Vikineswary, 2002; Ouhdouch et al., 2001) and extracellular hydrolytic enzymes (Mukherjee and Sen, 2006; Prapagdee, 2008; Valois et al., 1996). The exploration of new habitats plays an important role in search of new microbes possessing potentials to produce novel metabolites and is required urgently to counter the threats posed by the fast emerging phenomenon of antibiotic resistance (Shiburaj 2003). The present investigation was planned to optimize the production and characterization of antifungal compound produced by novel Streptomyces sp. SS12 (Singh et al., 2012).
MATERIAL AND METHODS
The Strain The bacterial strain was isolated from a pretreated soil sample collected from the terrestrial soil of western U.P., India, maintained on nutrient agar slants at 4?C, and also stored as glycerol stocks at −20?C. The strain was identified as Streptomyces sp. SS12 based on 16S rDNA sequence analysis (GenBank accession number AY426610) and BIOLOG biochemical profiling (Singh et al., 2012). Optimization of culture parameters for the production of antifungal antibiotic from Streptomyces sp. SS12 in SmF Selection of suitable antibiotic production medium The submerged fermentation was carried out in ten different culture media (M1- M10) to select preeminent production medium (Table 1). The activity of cell free fermented broth (permeate of 0.22? syringe filter) was evaluated after 48h by well diffusion method. Further various physiochemical parameters were optimized for the production of antimicrobial antibiotic in selected medium. Optimization of incubation period Fermentation broth was incubated for different time intervals (12, 24, 48, 72, 96, 120 and 144h) to find the optimal incubation period for antibiotic production. Optimization of various chemical parameters To find out the best suited carbon source the medium was supplemented with 1% (w/v) of different carbon sources such as monosaccharides (glucose, fructose), disaccharides (lactose, maltose), polysaccharide (starch), and alcohol (glycerol) followed by incubation at 28?C for 48h. The concentration of selected carbon source was optimized by supplementing the basal medium with various levels of glycerol (0.5, 1.0, 2.0, 3.0 and 4.0% v/v). The bacterial strain was grown in a set of basal media (with optimized level of glycerol) prepared by substituting beef extract with nitrogen equivalent of ammonium chloride, yeast extract, soybean meal, peptone and casein and incubated at 28?C for 72h in orbital shaker. The concentration of selected nitrogen source was optimized by growing the strain at various levels of nitrogen (2.0, 4.0, 6, 8.0 and 10.0 g/l) in basal medium.
Effect of various physical parameters on the production of antifungal antibiotic.
To study the effect of pH on the production of antifungal antibiotic, the bacterial strain was cultivated in the medium of varied pH (0.1M citrate buffer for pH 5.0; 0.1M phosphate buffer for pH 6.0 to 8.0; 0.1M glycine-NaOH buffer for pH 9.0). The effect of temperature was assessed by incubating the inoculated flasks at different temperatures (30, 35, 37, 40, 45 and 50?C). Rate of agitation was optimized by incubating the flasks at the rpm of 100-300 (100, 200, 250 and 300). Extraction of antimicrobial metabolites Organic solvents including ethyl acetate, petroleum ether, chloroform, benzene and xylene were used to determine the ideal solvent for extraction of the antibiotic from the culture supernatant (Busti et al., 2006). The cell free broth was mixed vigorously with the solvent and centrifuged at 5000 rpm for 10min to separate the phase antimicrobial compound. The solvent was evaporated to dry in water bath at 80?C and the residues obtained were used for assay. Characterization of partially purified antimicrobial compound Thermo stability of the antibiotic Thermo stability of the antifungal antibiotic was determined by exposing 1ml of suitably diluted, filter sterilized crude sample to 60, 80 and 100?C for 1, 2, 4, 6 and 8h. An untreated antibiotic sample (same dilution) was used as the control. These pre incubated antibiotic samples were used to determine their residual activity against the test organisms.
Effect of autoclaving on activity 1ml of suitably diluted crude antibiotic sample was taken in 2 sets of micro centrifuge tubes. Each set was autoclaved at 121?C, 15psi for 15min and 115?C, 10psi for 20min. Untreated antibiotic sample was treated as the control. pH stability of the antibiotic The pH stability of the partially purified antibiotic was studied by preparing antibiotic dilutions (5x) in buffers of pH 4.0, 7.0 and 9.0 and incubating them for 1, 2, 4, 6, 8 and 24h. These pre incubated antibiotic samples were used for determining the residual activity against the test organisms. Determination of fungi static or fungicidal nature of antibiotic To know the fungicidal or fungistatic nature of antibiotic, fungal spores and mycelia (Aspergillus sp.) were suspended in 500µL of potato dextrose broth (PDB) containing 10µL of undiluted partially purified culture filtrate of Actinomycetes strain SS12 and incubated for 12h at 30?C. PDB without antibiotic was served as control.
Effect of different concentrations of antibiotic on spore germination
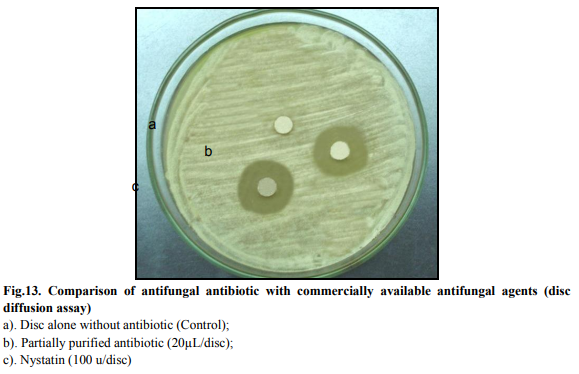
A series of test tubes containing 1ml fungal spore suspension (9x105 spores/ml) were supplemented with different concentrations of the culture filtrate (ranging from 0.5% to 5.0%). These were incubated at room temperature for 30min and 60min. After this pretreatment with antibiotic, 10μL sample of each spore suspension was placed on nutrient agar plates and incubated at 30?C and a plug was cut out and observed under microscope at various time intervals (1, 2, 3, 4, 5 and 6h). Untreated fungal spore suspension was used as the control and 100% spore germination time was noted. A comparative study of spore germination time and germ tube morphology, in control and test set-up, was conducted microscopically. Comparison of antibiotic with commercially available antifungal compounds The activity of antibiotic produced by Streptomyces sp. SS12 and commercially available preparations of antifungal antibiotics (Nystatin) was tested and compared by disc diffusion method. For agar disc diffusion assay, commercially available discs of Nystatin (100 units/disc), were used.
RESULTS AND DISSCUSSION
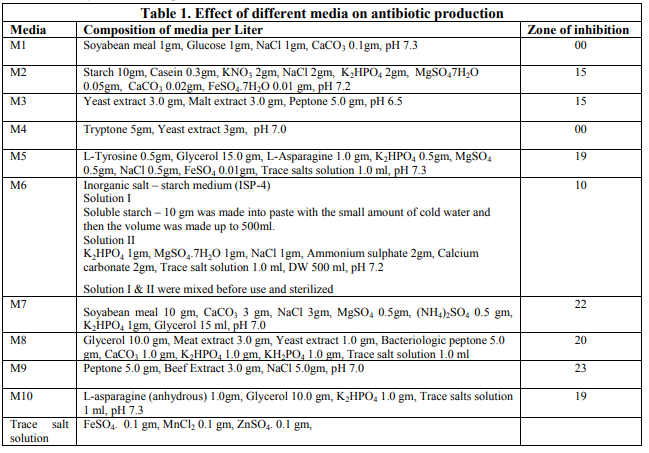
Optimization of medium component for antibiotic production Selection of suitable antibiotic production medium
Among the media tested for antimicrobial production M9 gave highest production level, which was reflected from a larger zone (25mm) while production was not detected in M1 and M4 (0mm) (Fig. 1). This may be due to the fact that M9 medium contains certain components that favors good bacterial growth and antibiotic production. The contribution of certain media components towards increasing antibiotic production, in submerged batch culture, has been reported for natamycin production by Streptomyces natalensis (Farid, 2000). Similarly Oskay (2011) showed that the activity of actinomycete isolates could be increased or decreased remarkably under different cultural conditions.
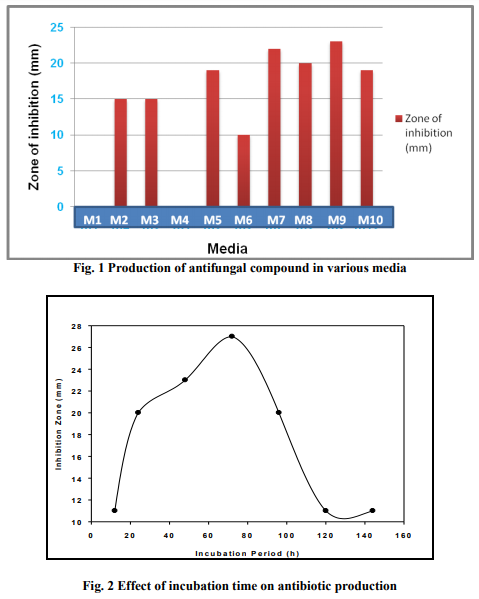
Effect of incubation period on antibiotic production
The antifungal activity of the culture filtrates of Streptomyces sp. SS12, was detectable after 12h of incubation and it enhanced with time. The antifungal activity was substantially high after 72h of incubation, and thereafter, no further increase was recorded (Fig. 2).
Optimization of various chemical parameters for antibiotic production
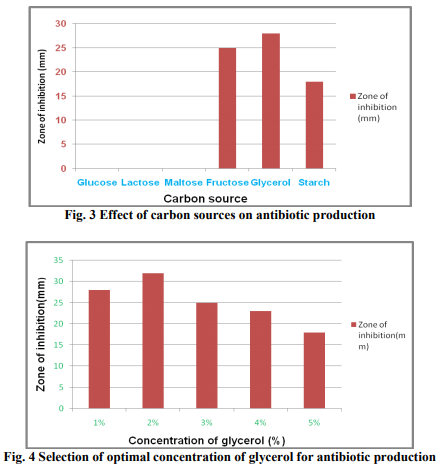
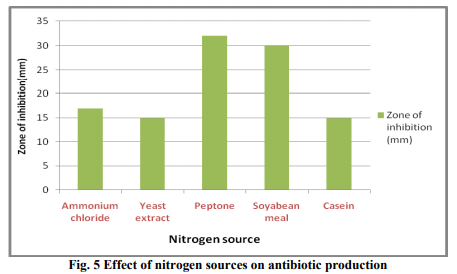
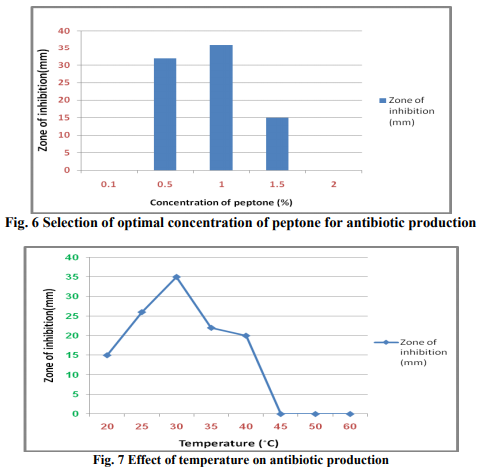
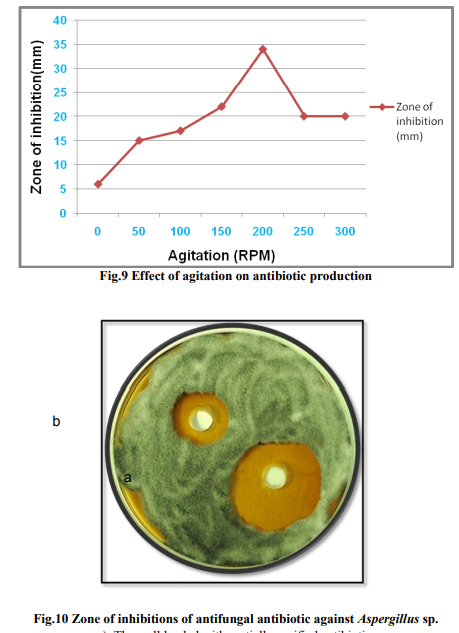
In order to design the effective medium, the roles of different carbon and nitrogen sources were evaluated for their impact on bioactive metabolite production. Glycerol was found to be the most appropriate carbon source (Fig. 3 & 4). The results were in accordance with Streptomyces sp. (Hague et al., 1995), where antibiotic production attains optima with glycerol. As glycerol was found best suited carbon source for bioactive metabolite production by the strain, different levels of glycerol (1~5%) were taken to determine the optimal concentration for bioactive metabolite production. 2% of glycerol supplemented in the medium promoted the highest antibiotic production. A fall in antibiotic production was observed at still higher concentrations of glycerol. These results are in line with those obtained by Mustafa Oskay, (2009). Hassan et al. (2001) found similar results with Streptomyces violatus in batch cultures. Nitrogen sources such as peptone, yeast extract and (NH4)2HPO4 have been reported to play important role in antifungal antibiotic production by Bacillus sp. (Fiddaman and Rossal, 1994). Media containing nitrogen sources such as ammonium chloride, yeast extract, peptone, soybean meal and casein were tested for antibiotic production (Fig. 5). Highest zone of inhibition was observed when medium was supplemented with peptone. Growth in the medium supplemented with 1% of peptone was highest after 72h of incubation and it decreased constantly with further increase in peptone concentration (Fig. 6). Effect of various physical parameters on the production of antifungal antibiotic Antibiotic production was increased with increase in pH of the buffered medium from 5.0 to 7.0, reaching a peak at 7.0. Further increase in pH beyond 7.0 causes a decline in antibiotic production (Fig. 8). The antibiotic production increased with increase in temperature, reaching a peak at 30?C. With further rise in temperature, antibiotic production decreased, and at 45?C antibiotic activity could not be detected (Fig. 7). The strain grew well at moderate temperatures and the growth was very sparse at 50?C. It has been noticed that the initial pH of the medium has a great influence on antibiotic production by actinomycetes (Tarasova et al., 1977). Maximum growth and antibiotic production were achieved at 200rpm. The growth was poor at low rpm (Fig. 9) and very less antibiotic production was observed in static conditions.
Characterization of the partially purified antibiotic
Thermo stability of the antifungal antibiotic
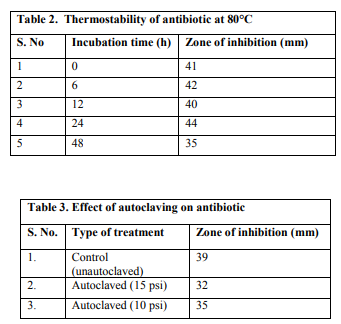
The antifungal compound produced by SS12 was found stable up to 24h at 80?C. However, a marginal decrease in the antifungal activity was observed after 24h (Table 2).
Effect of autoclaving on antifungal activity of antibiotic
A combined temperature and pressure treatment by autoclaving did not affect the stability and activity of the antifungal antibiotic (Table 3).
pH stability of the antifungal activity
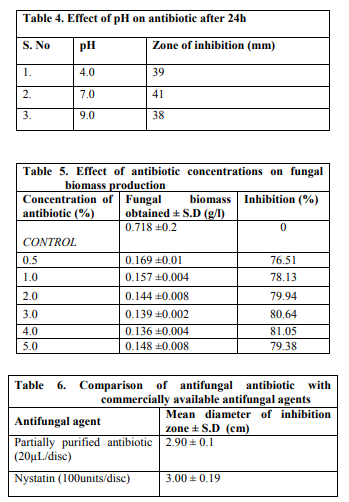
The antibiotic was stable at pH 4.0, 7.0 and 9.0 for upto 24h (Table 4). The pH alterations did not affect the antifungal activity of the antibiotic against Aspergillus sp.
Mode of action of antibiotic
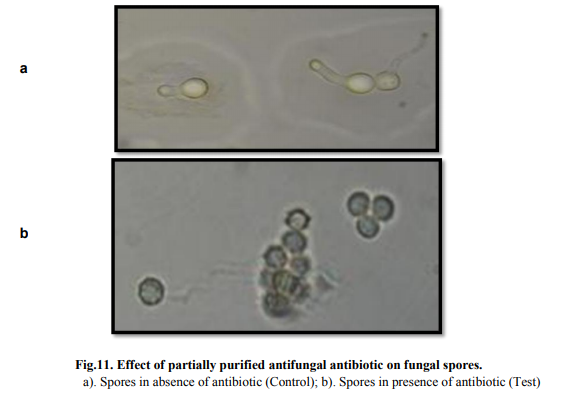
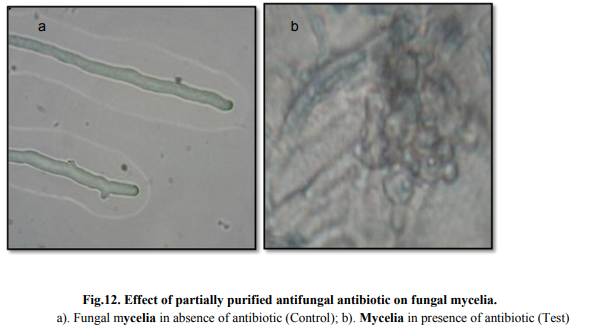
On microscopic observation complete inhibition of spore germination was observed (Fig. 11). Morphological alteration in mycelia was observed in compare to control under the light microscope (Fig 12). Effect of different concentrations of antibiotic on biomass production Concentrations of crude antibiotic as low as 0.5% caused 70% growth inhibition as compared to the control. An increase in concentration of antibiotic upto 5% concentration did not show substantial increase in fungal growth inhibition (Table 5).
Effectiveness of antifungal antibiotic produced by Actinomycetes SS12 vis-a-vis some commercially available antifungal agents
The antifungal activity of the antibiotic was compared with Nystatin using agar disc diffusion technique. The antifungal activity was studied against Aspergillus sp. nystatin disc (100units/disc) showed comparable inhibition zone to that of partially purified antifungal antibiotic (Table 6 and Fig. 13).
CONCLUSION
Optimization of process parameters resulted in 1.6 fold enhancement of antifungal antibiotic production. The bioactive compound showed stability and activity at broad range of temperature and pH. Microscopic observation of treated mycelia (Aspergillus sp.) showed significant distortion of mycelia while spore was not able to germinate in presence of antibiotic. As the compound showed comparable activity with commercially available antifungal (nystatin), hence, could be potential candidate for commercialization after established non-toxic nature.
ACKNOWLEDGEMENTS
Authors acknowledge the great help received from the scholars whose articles cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. Authors are grateful to IJCRR editorial board members and IJCRR team of reviewers who have helped to bring quality to this manuscript.
References:
1. WHO 2012: Department of Communicable Disease Surveillance and Response: WHO Global Strategy for Containment of Antimicrobial Resistance. WHO/CDS/DRS/2001 1.2 [http://www.who.int/csr/resources/publicati ons/drugresist/en /EGlobal_Strat.pdf].
2. Xu SX, Shen JL, Tang XF, Feng B. Newer antifungal agents for fungal infection prevention during hematopoietic cell transplantation: a meta-analysis. Transplantation Proceedings 2013; 45(1): 407-414.
3. Berdy J. The discovery of new bioactive microbial metabolites: screening and identification. In: Bushell ME, Grafe U (eds) Bioactive metabolites from microorganisms, Elsevier Science Publications, Amsterdam. 1989. pp. 3-25.
4. Busti, E., P. Monciardini, L. Cavaletti, R. Bamonte, A. Lazzarini, M. Sosio, and S. Donadio. Antibiotic-producing ability by representatives of a newly discovered lineage of actinomycetes. Microbiol. 2006, 152:675-683.
5. Farid, M.A., el-Enshasy, H.A., el-Diwany, A.I. and el-Sayed el-S.A. Optimisation of the cultivation medium for natamycin production by Streptomyces natalensis J. Basic Microbiol, 2000. 40(3): 157-166.
6. Fiddaman, P.J. and Rossall, S. Effect of substrate on production of antifungal volatiles by Bacillus subtilis J. Appl. Bacteriol. , 1994. 76(4): 395-405.
7. Fukai T, Yonekawa M, Hou AJ, Nomura T, Sun HD, Uno J. Antifungal agents from the roots of Cudrania cochinchinensis against Candida, Crytococcus and Aspergillus species. J. Nat. Prod., 2003. 66: 1118-1120.
8. Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinic experience. Rev. Infect. Dis., 1990. 12: 308- 329.
9. Getha K, Vikineswary S. Antagonistic effects of Streptomyces violaceusniger strain G10 on Fusarium oxysporum f. sp. cubense race 4: Indirect evidence for the role of antibiosis in the antagonistic process. J. Ind. Microbiol., 2002. Biotechnol., 28: 303-10.
10. Hague, S.F., S.K. Sen and S.C. Pal. Nutrient optimization for production of broad spectrum antibiotic by streptomyces antibioticus SR 15. 4. Acta- Microbial. Immunol. Hune. 1995. 42 (2): 155-162.
11. Hassan MA, El-Naggar MY, Said WY. Physiological factors affecting the production of an antimicrobial substance by Streptomyces violatus in batch cultures. Egypt. J. Biol. 2001. 3: 1-10.
12. Mukherjee G, Sen SK. Purification, Characterization, and antifungal activity of chitinase from Streptomyces venezuelae P10. Curr Microbiol. 2006. 53: 265-9.
13. Mustafa Oskay. Antifungal and antibacterial compounds from Streptomyces strains. African Journal of Biotechnology, 2009. Vol. 8 (13), pp. 3007-3017. 14. Oskay M. Effects of some environmental conditions on biomass and antimicrobial metabolite production by Streptomyces sp., KGG32. Int J Agric Biol., 2011. 13:317-24.
15. Ouhdouch Y, Barakate M, Finance C. Actinomycetes of Moroccan habitats: Isolation and screening for antifungal activities. Eur. J. Soil Biol., 2001. 37: 69-74.
16. Prapagdee B, Kuekulvong C, Mongkolsuk S. Antifungal Potential of Extracellular Metabolites Produced by Streptomyces hygroscopicus against Phytopathogenic Fungi. Int. J. Biol. Sci., 2008. 4: 330-337.
17. Shiburaj, S. Screening, isolation and characterization of an antibiotic producing Actinomycete Streptomyces setonii 19NRA1 (Ph.D thesis), 2003. University of Kerala.
18. Singh S, Rawat A, and Sharma DC. 2012. Antifungal antibiotic production by Streptomyces sp. isolated from soil. International Journal of Current Research and Review, 4 (24), 07 -16.
19. Tarasova, S.S., V.V. Biryukov and V.G. Makarevich. Double-substrate equations of kinetics of Actinomyces aureofaciens growth and tetracycline biosynthesis. Antibiotiki. 1977. 22(44): 291-297.
20. Valois D, Fayad K, Barasubiye T, Garon M, Dery C, Brzezinski R, Beaulieu C. Glucanolytic actinomycetes antagonistic to Phytophthora fragariae var. rubi, the causal agent of raspberry root rot. Appl. Environ. Microbiol. 1996. 62: 1630-5.

|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License