IJCRR - 5(12), June, 2013
Pages: 57-60
Date of Publication: 28-Jun-2013
Print Article
Download XML Download PDF
STUDY OF MICROBIAL FLORA IN PATIENTS WITH INDWELLING CATHETER
Author: Manish N., Tankhiwale N. S.
Category: Healthcare
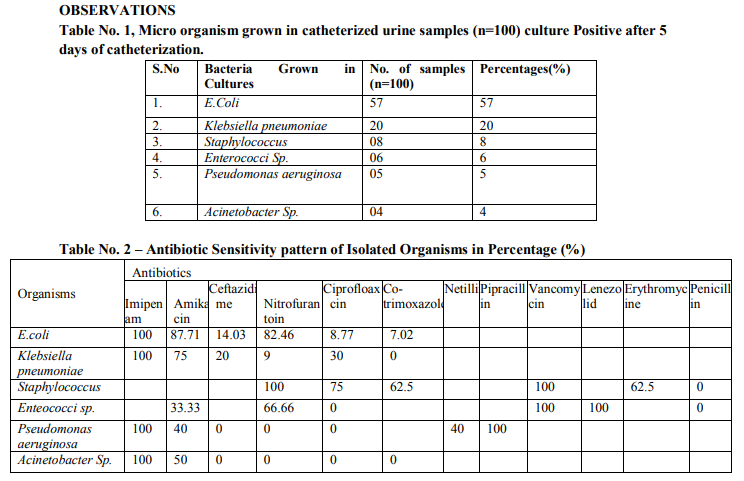
Abstract:Introduction: - Catheter associated urinary tract infections are common nosocomial infections, 80% cases of UTI are due to use of catheters. The incidence of becteriuria in catheterized patient is directly related to the duration of catheterization. E.coli, Proteus, Pseudomonas, Klebsiella, Serratia, Staphylococcus, Enterococci and Candida Sp. Are the common micro-organisms causing this infection? Aims and Objective: - The present study is to determine the microbiological profile and the sensitivity pattern which can cause Catheter Associated Urinary Tract Infections. Materials and Method: - The study was conducted in 100 adult patients, whom an indwelling Foley's catheter was inserted in AVBR Hospital during August 2011 to August 2012, in different medical wards, surgery wards and ICU. The catheterized urine sample was collected after catheterization on 5th day onwards on indwelling catheterization. Approximately 3 ml of urine was taken in sterilized container with the sterile precautions. The urine sample microscopy for pus cells, other abnormalities, gram staining was done and inoculate urine in culture medium. The final reading was done after 18 to 24 hrs. Of incubation of culture plates at 37oC. Antibiogram testing was done by Kirby-Bauer disk diffusion technique. Result and Conclusion :- The most common organism colonizing and causing catheter associated urinary tract infection as per observation were found to be E.Coli (57%) followed by Klebsiella Sp.(20%), Staphylococcus (8%), Enterococcus Sp. (6%), Pseudomonas aeruginosa (5%) and Acinetobacter Sp. (4%). Frequent cleanliness of catheter, area to avoid contamination and colonization of microbial flora, it is recommended to change the catheter every 5th day.
Keywords: Catheter associated urinary tract infection, becteriuria, E.Coli
Full Text:
INTRODUCTION
Catheter associated urinary tract infections are common nosocomial infections, 80% cases of UTI are due to use of catheters.1 Fifteen to twenty five percent of patient in hospitals, need catheterization.2 Factors that have increased the risk of catheter associated urinary tract infections are prolonged catheterization, severe underlying illness, disconnection of catheter and drainage tube, faulty catheter care and lack of systemic antibiotics therapy.3 About 1% to 48% of hospitalized patient with indwelling catheters still acquire the infection.2 The incidence of becteriuria in catheterized patient is directly related to the duration of catheterization.4 Urethral catheter is a major predisposing factor in the development of nosocomial UTI and catheter- associated bacteremia.5 Most nosocomial UTI can be benign but a systemic complication which is gram-negative septicemia can develop in 30-40% of patients. E.coli, Proteus, Pseudomonas, Klebsiella, Serratia, Staphylococcus, Enterococci and Candida Sp. are the common micro-organisms causing this infection. Many infecting strains display markedly greater antibiotics resistance than organisms that cause community-acquired Urinary tract infection.7 Study of microbial flora in these patients can prevent establishment of UTI and further kidney damage. Antibiotic sensitivity testing will help the clinician to give proper treatment.6
AIMS AND OBJECTIVE
The present study is undertaken to determine the microbiological profile and the sensitivity pattern of the strains which can cause Catheter Associated Urinary Tract Infections.
MATERIAL AND METHOD
The study was conducted in 100 adult patients, whom an indwelling Foley’s catheter was inserted in AVBR Hospital during August 2011 to August 2012. The study was undertaken in patients catheterized and admitted in different medical wards , surgery wards and ICU. The catheterized urine sample was collected after catheterization on 5th day onwards on indwelling catheterization. Sample was collected by 24- gauge needle with all sterile precautions and urine was brought to microbiology lab within 1 hour of collection for further processing. Approximately 3 ml of urine was taken as a sample in sterilized container with the sterile precautions with sterilized syringe. The microorganism growth was seen only after 5th day of catheterization. The urine sample microscopy for pus cells, other abnormalities and gram staining was done. Standard loop was used for inoculating urine in culture medium. The urine was subjected to culture on Blood agar, MacConkey agar, CLED (Cystine Lactose Electrolyte Deficient Medium),TSI and Nutrient agar. The final reading was done after 18 to 24 hrs. of incubation of culture plates at 37oC. The identification of micro-organisms was done by the colony characters, Gram staining, morphology, biochemical reactions as per standard text book (Practical Medical Microbiology, Mackie & McCartney, 14th edition). Colony count >105 cfu/ml was considered as significant. Antibiogram testing was done by Kirby-Bauer disk diffusion technique in MuellerHinton medium.
DISCUSSION
The result of the microbiologic profile in this study is similar to most reported studies. E.Coli still being the most common pathogen (57% of cases) followed by Klebsiella pneumoniae(20%), Staphylococcus(8%), Enterococcus Sp.(6%), Pseudomonas aeruginosa (5%) and Acinetobacter Sp.(4%). In a study conducted by Poudel C.M., Baniya G. Department of Internal Medicine and Department of Microbiology, TUTH, Katmandu the majority of organisms belonged to E.Coli (40.77%),Klebsiella pneumoniae (11.11%), Enterococcus Sp.(11.11%),Pseudomonas (11.11%),Acinetobacter Sp. (3.7%) are all most similar. Present study is comparable to those workers. The most common organism colonizing and causing catheter associated urinary tract infection as per observation were found to be E.Coli (57%) followed by Klebsiella Sp.(20%), Staphylococcus (8%), Enterococcus Sp. (6%), Pseudomonas aeruginosa (5%) and Acinetobacter Sp. (4%). E.Coli are found to be sensitive to Amikacin (87.71%), Nitrofurantoin (82.46%) Ceftazidime (14.03%), least sensitive to Ciprofloxacin (8.77%) and Co-trimaxazole (7.02%) and 100% sensitive to Imipenem. Klebsiella Sp. are found to be sensitive to Amikacin (75%),Ciprofloxacin (30%), least sensitive to Nitrofurantoin (9%) and 100% sensitive to Imipenam and 100% resistance to Co-trimoxazole. Staphylococcus are found to be sensitive to Ciprofloxacin (75%), Co-trimoxazole (62.5%), Erythromycine (62.5%) and 100% sensitive to Nitrofurantoin and Vancomycin and 100% resistance to Penicillin. Enterococcus Sp. are found to be sensitive to Nitrofurantoin (66.66%), Amikacin(33.33%) and 100% sensitive to Vancomycin and Lenezolid and 100% resistance to Penicillin and Ciprofloxacin. Pseudomonas aeruginosa are found to be sensitive to Amikacin (40%), Netillin (40%) and 100% sensitive to Imipenam and Pipracillin and 100% resistance to Ceftazidime, Nitrofurantoin and Ciprofloxacin. Acinetobacter Sp. are found to be sensitive to Amikacin (50%) and 100% sensitive to Imipenam and 100% resistance to Ceftazidime, Nitrofurantoin, Ciprofloxacin and Cotrimoxazole.
CONCLUSION AND RECOMMENDATIONS
- Frequent cleanliness of catheter, area to avoid contamination and colonization of microbial flora, it is recommended to change the catheter every 5th day.
- Proper aseptic precaution to be taken while collection of samples by syringe.
- Microbial flora should be evaluated in catheterized patients through out.
- The antibiotic sensitivity report should be referred before giving treatment, if infection is established.
ACKNOWLEDGEMENT
We acknowledge the great help received from the scholars whose articles cited and included in references of this manuscript. We are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. We are grateful to IJCRR editorial board members and IJCRR team of reviewers who have helped to bring quality to this manuscript.
References:
1. Alavaren HF, Lim JA, Antonio-Velmonte M, et al. Urinary Tract Infection in Patients with Indwelling Catheter. Phil J Microbiol Infect Dis 1993; 22(2):65- 74.
2. Billote-Domingo K, Mendoza MT and Torres TT. Catheter-related Urinary Tract Infections: Incidence, Risk Factors and Microbiologic Profile. Phil JMicrobiol Infect Dis 1999; 28(4):133-138.
3. Wenzel R P and Edmond M B. The Impact of Hospital- Acquired Bloodstream Infections. Emerging Infectious Diseases 2001; 7(2):174-177.
4. Saint S, Lipsky BA, and Goold SD. Indwelling Urinary Catheters: A One-Point Restraint? Annals of Internal Medicine 2002; 137(2):125.
5. Mandell, Douglas, Bennet. Principles and Practice of Infectious Diseases. 3rd ed 1990. pp 2205-2215.
6. Platt R, Polk BF, Murdock B, et al. Mortality associated with nosocomial urinary-tract infection NEJM 1982; 307(11):637-642.
7. Walter ES. Urinary tract infections and pyeloneephritis. Harrison’s principles of internal medicine. 16th edition 2004; Volume II; 1715-21.
8. Jain P, Parada JP, David A, et al. Overuse of the indwelling urinary tract catheter in hospitalized medical patients. Arch Intern Med 1995; 155(13): 1425-1429.
9. Dickson G M and Bisno A. Infections Associated with Indwelling Devices: Infections Related to Extravascular Devices antimicrobial agents and chemotherapy. American Society for Microbiology 1989; 33(5): 602-607.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License