IJCRR - 9(12), June, 2017
Pages: 11-15
Date of Publication: 24-Jun-2017
Print Article
Download XML Download PDF
Growth and Characterization of NaCl doped Organometallic L-Asparagine Cadmium Chloride Monohydrate (LACCM) Crystals
Author: S. Mugunda Kumari, N. Thangaraj, R. Rakhesh, N. Joseph John
Category: General Sciences
Abstract:Single crystals of sodium chloride (NaCl) doped organometallic nonlinear optical material L-asparagine cadmium chloride monohydrate (LACCM) were successfully grown by slow evaporation method at room temperature. Grown crystals were characterized by single crystal and powder X-ray diffraction analysis, scanning electron microscopy, energy-dispersive analysis by X-ray and thermal studies. The second harmonic generation was confirmed by the Kurtz and Perry powder technique. The presence of various functional groups was identified from FTIR spectral analysis. The mechanical strength of the grown crystal has been determined with the aid of Vickers hardness test. The second harmonic efficiency and dielectric constant increases significantly due to the introduction of sodium ions into the lattice of l-aspargine cadmium chloride crystal, so that this crystal is a potential material for nonlinear frequency conversion.
Keywords: Nonlinear optic material, Crystal growth, XRD, FTIR, Thermal studies
Full Text:
Introduction
In recent years, tremendous efforts have been devoted to synthesize organometallic complex second order nonlinear optical (NLO) materials capable of frequency conversion into visible and ultraviolet (UV) wavelengths. Such materials are widely used in device fabrications relating to telecommunications, optical computing, optical disk storage, and optical information processing. These materials have the potential for combining the high optical nonlinearity and chemical flexibility of organics with the physical ruggedness of inorganics [1–5]. Organometallic compounds differ from most organic NLO materials by charge transfer transitions i.e metal-to-ligand and ligand-to-metal. These NLO metal complexes exhibit donor (π-conjugate bridge) and acceptor (D-π-A) structures [6].
In case of metal organic co-ordination complexes the organic ligand is usually more dominant in the nonlinear optical (NLO) and dielectric effects, The metallic part focus in on group II B metals (Zn , Cd and Hg).These compounds usually have high transparency in UV region , because of their closed d10 shell. Potential NLO materials like bis thiourea cadmium chloride (BTCC), triallyl-thiourea cadmium chloride (TATCC)[7] are examples of this approach.
The amino acids are the famous organic materials. Play a vital role in the field of nonlinear optical crystal growth. Many members of natural amino acids individually exhibiting the nonlinear optical properties because they have a donor NH2 and acceptor COOH group and the intermolecular charge transfer is also possible. Especially natural amino acids such as Arginine, alanine, lysine and γ glycine are evidently showing NLO property because of additional COOH group in first and NH2 group in second. Therefore mixing of amino acid with already known organic, inorganic or semi-organic NLO materials may improve their NLO and ferroelectric properties. The literature survey confirmed the studies on improved second harmonic generation, thermal, and opto-electric properties of crystals grown by mixing equimolar ratios of amino acids L-alanine, L-arginine with malic acid, oxalic acid, nitric acid and acetic acid [8-13]. The title compound L-Asparagine cadmium chloride monohydrate (LACCM) was investigated by many authors [14,15]. In our present work aiming to improve second harmonic efficiency, sodium chloride doped the single crystals of LACCM were grown by slow evaporation technique and the effect of Na+ ion on structural, thermal and dielectric properties were analyzed in detail.
Materials and methods
Synthesis and growth of NaCl doped LACCM
AR grade L-aspargine, Cadmium chloride monohydrate, Sodium chloride purchased from Merck India and double distilled water were used for the growth of NaCl doped LACCM crystals. L-aspargine and Cadmium chloride were taken in equimolar ratio. Calculated amounts of the reactants were thoroughly dissolved in double distilled water. Then, it was mixed with continuous stirring for about 2 h using magnetic stirrer with hot plate. One mole percentage of sodium chloride was added to the supersaturated aqueous solution prepared in a 100 ml beaker (corning glass vessel) and allowed to equilibrate at the desired temperature. The crystals were grown in the unstirred condition by slow evaporation technique [16-22]. The temperature and volume were kept constant, respectively at 30°C and 20ml for all the crystal growth experiments.
The beakers were covered tightly with polythene covers. Small holes were made on the cover for proper evaporation of the solvent. The whole setup was kept in a dust-free area and closely monitored. The solution loses particles which are weakly bound to other components and, therefore, the volume of the solution decreases. An excess of a given solute is established by utilizing the difference between the rates of evaporation of the solvent and the solute. Normally,
the vapor pressure of the solvent above the solution is higher than the vapor pressure of the solute and, therefore, the solvent evaporates more rapidly and the solution becomes supersaturated. It is sufficient to allow the vapor formed above the solution to escape freely into the atmosphere. Homogeneity of the solution is expected to be maintained during crystal growth due to normal convection process of the liquid. Small crystals appeared in the beginning due to slow evaporation and grew larger (up to about 2 cm in size) in considerable finite time of about 6 hrs. After the completion of growth, crystals were harvested. Good quality optically transparent large size crystals were selected for carrying out the measurements.
Characterization
FTIR studies of the grown crystals were analyzed by Fourier Transform Infra Red spectrometer model SPECTRUM RXI make PERKIN. The single crystal X-ray diffraction studies of the grown crystals were carried out using BRUKER KAPPA APEX II model single crystal X-ray diffractometer with MoKα (λ = 0.717 Å) radiation. Powder X-ray spectrum was obtained by PANalytical X’Pert Pro Powder X’Celerator diffractometer. The optical transmittance spectrum was recorded in the range of 190-1100 nm, using Lamda35 Perkin Elmer make UV-Vis-NIR spectrometer. Micro hardness studies have been carried out using a SHIMADZU HMV-2T model hardness tester. The applied load was varied from 5 to 100 g with a constant indentation time of 15 seconds in each case. The hardness profile was studied by plotting the variation of hardness number (HV) with applied load (P). The NLO test of LACCM crystals were evaluated by the Kurtz and Perry powder technique [23] using a Q-switched, mode locked Nd : YAG laser emitting 1.06μm, 8 ns laser pulses with spot radius of 1 mm. DSC measurements were done by Mettler Toledo DSC 822E model calorimetry.
Result and discussion
Crystals with regular shape and size of about 14×11× 5 mm3 were harvested within 15 to 20 days. The photograph of the grown LACCM crystals is displayed in Fig. 1. The external appearance or morphology of the grown crystals seems to be polyhedron in shape. Morphology of crystals changes when growth conditions such as growth media, temperature and addition of impurities are altered. Since the growth temperature has not been completely kept constant during the growth of the crystals in the present work, there are morphological changes in the grown crystals.
FTIR analysis
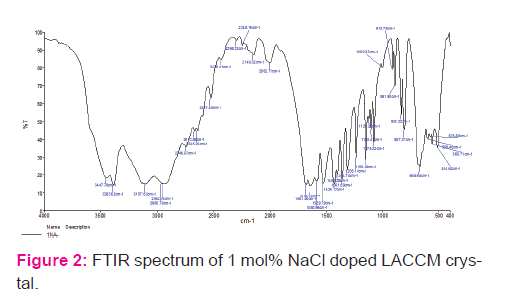
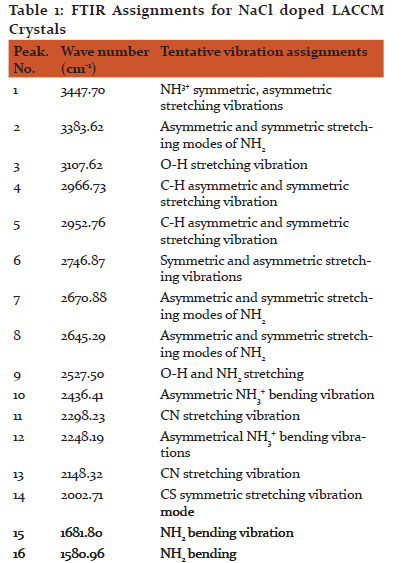
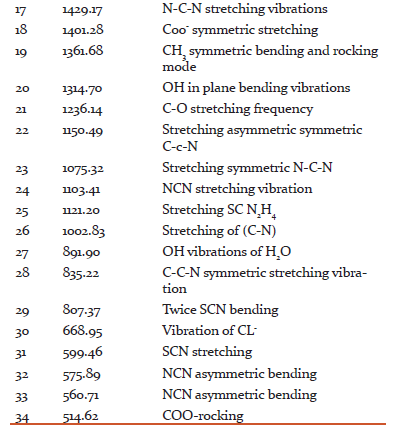
The formation of functional groups was confirmed by using SPECTRUM RXI make PERKIN FTIR spectrometer by KBr pellet technique with a scan range of 4000–400 cm−1 as shown in Fig. 2. Table 1 gives the assignments for all peaks. The peak around 3383–3107 cm−1 is due to NH stretching of NH2 vibration. The peak obtained at 2996 cm−1 is due to CH2 vibration of the amino acid. The NH3 + asymmetric vibration is observed at 1681 cm−1 and NH2 torsional oscillation at 514 cm−1. The peaks obtained at 1580 and 1429 cm−1 are due to asymmetric and symmetric vibrations of COO− [23]. The C–C–COO vibration occurs at 1236 and 1150 cm−1. The peak obtained at 891 cm−1 is due to C–C–N symmetric vibration. The peaks at 668 and 514 cm−1 indicates that COO− bending and COO− rocking vibration. These vibrations proved the presence of expected functional groups in the synthesized compound and in good agreement with the reported value [14].
Structural analysis
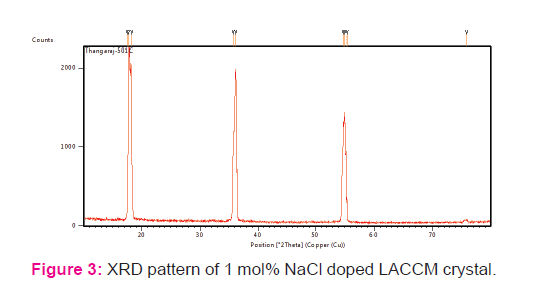
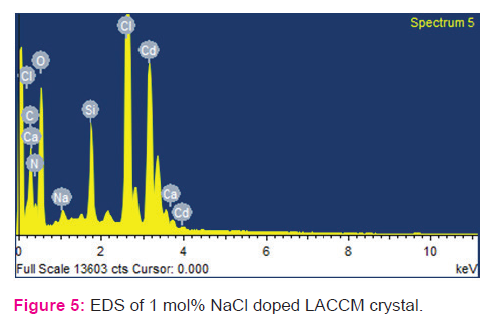
Crushed powder of LACCM crystal was subjected to powder X-ray diffraction analysis. The sample was scanned the wide range of 0–80? with a scan rate of 2?/min. The recorded X-ray pattern of LACCM is shown Fig 3. The prominent well defined sharp Bragg’s peak at specific 2θ angle reveals that the good crystalline nature of NaCl doped LACC crystal. Crystal size of 0.200 x 0.200 x 0.300 mm and wave length 0.71073 Å was used for single crystal XRD. The crystal system is orthorhombic and unit cell dimensions are a = 5.5801(12) Å, b = 9.804(3) Å, c = 11.804(3) Å, cell volume V=645.7(3) Å3 ,α = 90°, β = 90°, γ = 90°. The presence of sodium ions in LACCM doped with sodium chloride was confirmed by EDAX. The composition of the elements present in the sodium chloride doped LACCM crystals are displayed along with SEM image in fig. 4 & 5.
NLO test
The NLO property of the crystal was confirmed by the Kurtz and Perry powder technique[24]. The transmitted fundamental wave was passed over a monochromator, which separates 532 nm (second harmonic signal) from 1064nm and absorbed by a CuSO4 solution, which removes the 1064 nm light. The green light was detected by a photomultiplier tube and displayed on a storage oscilloscope. The powder SHG efficiency of the crystal is compared with KDP and it is found to be 0.98 times that of KDP due to the higher polarization of smaller ionic radii Na ions.
Microhardness test
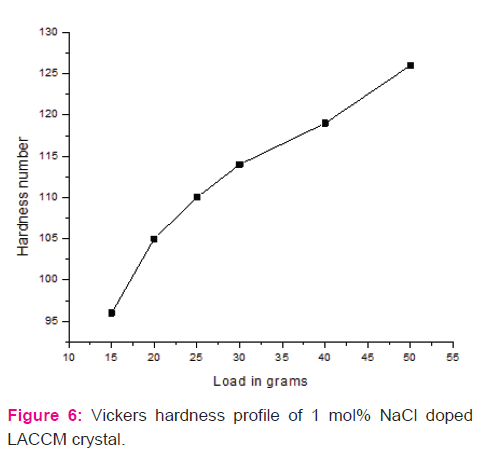
The Vickers hardness number (Hv) increases with respect to increase in load. The value of Hv is 38 and 70 for the loads 0.025 and 0.100 kg, respectively. The value of the work hardening coefficient (n) was estimated from the ratio of log p to log d (using the Mayer’s relation p=kdn). It is observed that the value of n was 1.1568 for the load p=0.100 kg. For hard materials the value of the work hardening coefficient(n) liesbetween1.0 and 1.6 [22] and hence it is concluded that sodium chloride doped LACCM crystal belongs to the category of hard materials.
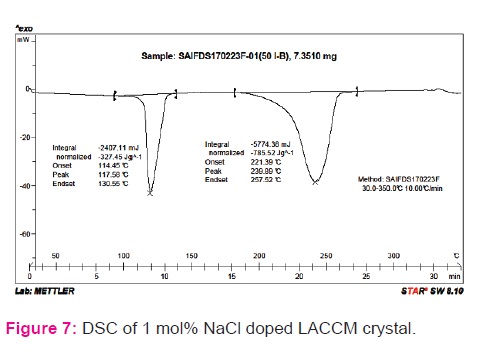
Thermal analysis
Thermal stability of the LACCM crystal was studied by DSC analyses. The recorded DSC spectra of the sample are shown in Fig. 7. From the DSC curve, the thermal stability of the sample is realized upto 400°C. Sodium chloride doped LACCM material shows loss in weight due to the molecules, which are loosely bounded to the central metal atom at 117-130°C and 239- 257 °C. These molecules break their bonds get detached and leave the complex as fragments from the coordinating sphere. Thermal resistance offered by sodium chloride doped LACCM has been observed upto 400 °C, which is attributed to high cohesive energy of the transition metal complex with strong bonding nature [25]. The binding energy of ionic crystal (sum of electrostatic and Van der Waals part of attractive interactions) explains the thermal resistance of the complex. Endothermic peak reveals that melting occurs at 118° C and subsequent exothermic peak represents the decomposition nature of the sample.
Conclusion
The organometallic NLO single crystals of sodium chloride doped L-aspargine cadmium chloride (LACCM) were grown by slow evaporation technique and characterized by X-ray diffraction (single crystal land power) studies. The FT-IR spectral analysis confirms the presence of functional groups in the compound. The presence of sodium impurity was confirmed by energy dispersive analysis by X-ray. The TG analyses how that the material has thermal stability up to 400°C. The NLO property is confirmed by SHG measurement. The micro-hardness study proved that this organometallic compound belongs to the category of hard materials.
Acknowledgement
The authors acknowledge STIC Cochin for SXRD and thermal studies, IISc Bangalore for SHG measurement, St. Joseph College Trichy for FTIR and microhardness studies, National College Trichy for SEM and EDAX spectrum and Alagappa University Karaikudy for PXRD studies


References:
1. Xu, D.; Yuan, D.R.; Zhang, N.; Hou, W.B.; Liu, M.G.; Sun, S.Y.; Jiang, M.H. Study of properties and structural features of some new organic and organometallic nonlinear optical crystals. J. Phys. 1993, D26, B230–B235.
2. Long, W.J. Organometallic compounds for nonlinear optics. The search for enlightenment. Angew. Chem. Int. Ed. Engl. 1995, 34, 21–38.
3. Jiang, M.H.; Fang, Q. Organic and semiorganic nonlinear optical materials. Adv. Mater. 1999, 11, 1147–1151.
4.N. Joseph John , Benita Jeba Silviya , P. Selvarajan, C. K. Mahadevan, Growth and characterization of Disodium hydrogen orthophosphate (DAHP) single crystals, Materials and Manufacturing Processes , 22, 379, (2007)
5. N. Joseph John, P. Selvarajan, C. K. Mahadevan, Studies on NaCl doped DSHP crystals, Materials and Manufacturing Processes, 23, 809, (2008)
6. S.D. Bella, Second-order nonlinear optical properties of transition metal complexes chem. Soc.Rev30,355, .(2001)
7. M.Jiang , Q.fang, Organic and semiorganic nonlinear optical materials, Adv. Mater11,1147, .(1999).
8. Martin Britto Dhas, S.A., Bhagavannarayana, G. Natarajan, S., Growth, HRXRD, Microhardness and Dielectric Studies on the NLO Material L-Alaninium Maleate, The open crystallography Journal, 1. (2008)
9.U. Karunanithi, , S. Arulmozhi, , J. Madhavan, Synthesis and Characterization of Pure and Doped L-Arginine Maleate Single Crystals , Journal of Appied Physics, 1, (2012).
10. R. Mohan Kumara., D. Rajan Babu, D.Jayaraman, R. Jayavel, K. Kitamura., Journal of Crystal Growth, 3, 275. (2005)
11. K. J. Arun, S. Jayalekshmi, Growth and Characterisation of Nonlinear Optical Single Crystals of L-Alaninium Oxalate, Journal of Mineral Materials Characterisation & Engineering, 8,8 , (2009).
12. P.Praveen Kumar, V.Manivannan, P.Sagayaraj, J. Madhavan, Growth and characterization of pure and doped NLO L-arginine acetate single crystals, Bulletin of Material Science, Vol. 32.,1857 (2009),
13. M Vimalan, TR Kumar, S Tamilselvan, P Sagayaraj, CK Mahadevan, Growth and properties of novel organic nonlinear optical crystal: l-alaninium tartrate (LAT), Physica B: Condensed Matter 405 (18), 3907-3913, (2008)
14. S. Masilamania,, P. Ilayabarathi, P. Maadeswaran, J. Chandrasekaran, K. Tamilarasan, Synthesis, growth and characterization of a novel semiorganic nonlinear optical single crystal: l-Asparagine cadmium chloride monohydrate, Optik, 123, 1304, (2012) 10.1016/j.ijleo.2011.07.063
15. Bikshandarkoil R. Srinivasan, On the existence of ‘L-asparagine cadmium chloride monohydrate’ crystal, Optik, 10.1016/j.ijleo.2013.12.082
16. N. Joseph John, P. Selvarajan , C. K. Mahadevan, Growth, Structural, Optical, Mechanical and Dielectric Characterization of Diammonium Hydrogen Phosphate (DAHP) single Crystals, Journal of Minerals and Material characterization and Engineering, 10, 15, 1379 (2011).
17. A.J. Jayaprakash Manoharan, N. Joseph John , P. Andavan, Effect of amino acid doping on the dielectric properties of triglycine sulphate (TGS) single crystals , Ind. J. of Sci. and Tech., 4,6, 688, (2011).
18. A. J. Jayaprakash Manoharan, N. Joseph John, P.Andavan, Journal of Experimental Sciences, 2(2) (2011), 33.
19. P. Sivaka, N. Joseph John, S. Perumal, Int. Journal of Engineering Research and Applications, 4, 7 (2014), 145.
20. P. Sivaka, N. Joseph John, S. Perumal, Int. res. J. Eng. And Tech., 3, 2 (2016), 1273.
21. N. Joseph John, Int. J. Current Sci. 8,11 (2016), 41068.
22. N. Joseph John, Int. J. Innov. Sci. and Res. 5,11(2016), 890.
23.R.M. Silverstein, G.C. Bassler, T.C. Morrill, Spectrometric Identification of Organic Compounds, fifth ed., Wiley, New York, 1991.
24. Kurtz S.K. and Perry T.T., A Powder Technique for the Evaluation of Nonlinear Optical Materials J. Appl. Phys, 39, 3798, (1968).
25.C. Razzetti, M. Ardonio, L. Zanotti, M. Zha, C. Paorici, Solution growth and characterisation of L-alanine single crystals, Cryst. Res. Technol. 37 (2002) 456
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License