IJCRR - 5(13), July, 2013
Pages: 51-57
Date of Publication: 17-Jul-2013
Print Article
Download XML Download PDF
EVALUATION OF SERUM LEVELS OF REDUCED GLUTATHIONE, GLUTATHIONE-S-TRANSFERASE AND NITRIC OXIDE IN BREAST CANCER PATIENTS UNDERGOING ADJUVANT CHEMOTHERAPY
Author: Charushila Y. Kadam, Subodhini A. Abhang
Category: Healthcare
Abstract:Background: Alteration in oxidative and nitrosative stress as well as antioxidant status is known to occur in carcinogenesis and during treatment of cancer with chemotherapeutic drugs. Objective: The objective of this study was to evaluate the serum levels of reduced glutathione (GSH) glutathione-s-transferase (GST) and nitric oxide (NO•) in post-operative stage II breast cancer patients undergoing adjuvant chemotherapeutic treatment. Material and Methods: Clinically and histopathologically proven 30 stage II breast cancer patients were selected for the present study. Blood was collected after mastectomy before start of 1st adjuvant chemotherapy cycle and 3 weeks after receiving 1st cycle of chemotherapy. 30 healthy controls were selected for comparison. Serum GSH, GST and NO• levels were estimated by spectrophotometric methods. Results: The serum level of reduced glutathione was significantly lower (P< 0.0004) whereas serum levels of GST and NO• were significantly higher (P< 0.0001) in post-operative stage II breast cancer patients before chemotherapy as compared to healthy controls. After 3 weeks of receiving 1st adjuvant chemotherapy cycle, significant decrease in the serum levels of GSH, GST and NO• was observed (P< 0.0001) as compared to levels before chemotherapy in these patients. Significant positive correlation was found between GSH and NO• (r=+0.96) and GSH and GST (r= +0.91) after chemotherapy. Conclusion: The findings of the study suggests that administration of chemotherapeutic drugs in adjuvant setting in breast cancer patients causes increase in oxidative stress as indicated by decreased levels of antioxidant glutathione. The observed decrease in levels of GST and NO• might be associated with decrease in levels of GSH in these patients after chemotherapy.
Keywords: Antioxidants, Oxidative stress, FEC, S-nitrosoglutathione.
Full Text:
INTRODUCTION
Breast cancer is the most common cancer in women and is a leading cause of cancer related deaths in women worldwide 1 . The etiology of breast cancer is multifactorial including age, obesity, oral contraception, diet, family history and prior history of benign breast disease 1, 2 . Though breast cancer can be detected at an early stage, in some patients death occurs due to metastasis and recurrence 3 . The breast cancer treatment mainly comprised of surgical intervention with chemotherapy, radiotherapy and endocrine manipulation, either in the neoadjuvant and/or adjuvant setting 4, 5 . Oxidative stress caused by increased free radical generation and/or decreased antioxidant level in cell has been suggested to play an important role in carcinogenesis and treatment of cancer with chemotherapeutic drugs 6, 7. Free radicals can cause damage to all major classes of biomolecules such as lipids, proteins and nucleic acids and causes changes in their structure and function 2, 6, 7. To combat the deleterious effects of these free radicals, cells have developed different antioxidant mechanisms consisting of enzymatic and non-enzymatic components 1, 2 . Reduced glutathione (GSH) is the most abundant thiol in cells which acts as an important antioxidant. GSH is capable of scavenging hydrogen peroxide and peroxynitrite 8, 9, 10. It may affect the bioactivity of NO• . It is the precursor of s-nitrosoglutathione (GSNO). GSNO can transnitrosate protein thiols, possibly changing protein functions 11. GSH has been shown to be required for maximal activity of inducible nitric oxide synthase (iNOS) in hepatocytes and macrophages 12, 13. Glutathione-s-transferase (GST) is a ubiquitous multifunctional enzyme involved in detoxification. GST catalyze the conjugation of GSH to electrophilic species and plays a central role in the defense against free radicals, lipid hydroperoxides and detoxification of potential alkylating agents including anticancer drugs 13,14. Nitric oxide (NO• ) is an inorganic free radical gas produced by nitric oxide synthase (NOS) using L-arginine 15. Nitric oxide (NO• ) is a polyfunctional molecule that controls the variety of processes such as: vasodilatation, neurotransmission, immunocytotoxicity, carcinogenesis, induction or inhibition of apoptosis, etc 16, 17, 18. Furthermore, it is well known that NOS activity increases in some invasive tumors 19. The role of NO• in tumor biology is still poorly understood 15. Elevated levels of NO• provides primary source of reactive nitrogen species like peroxynitrite 2, 15 . Peroxynitrite which may be cytotoxic by itself is much more reactive and causes diverse chemical reactions in biological systems including nitration of tyrosine residues of proteins, triggering lipid peroxidation, inhibition of mitochondrial electron transport and oxidation of biological thiol compounds 15. Changes in the serum levels of nitric oxide (NO• ), reduced glutathione (GSH) and glutathione-s-transferase (GST) have been reported previously in breast cancer patients 1, 2, 7, 14, 16, 17. However, there is little research regarding the changes in these parameters after chemotherapy. Therefore, the present study was undertaken to evaluate the serum levels of nitric oxide, reduced glutathione and glutathione-stransferase in post-operative stage II breast cancer patients receiving adjuvant chemotherapy treatment.
MATERIAL AND METHODS
30 female breast cancer patients diagnosed with invasive ductal/lobular carcinoma with stage II classified by Tumor-Node-Metastasis (TNM) system and proven by histopathological evidence were involved in this study. The patients were admitted in the Sassoon General Hospital, Pune [Maharashtra, India] and were of 30 - 75 years age (Mean age 52.5±13.42). 30 healthy and age matched female controls were selected for comparison. The study was approved by Institutional Ethical Committee [Ref No. BJMC/IEC/Pharmac/D1210137-39]. After obtaining prior written consent from healthy volunteers and breast cancer patients, 5ml of venous blood was drawn under aseptic precautions after mastectomy before the start of chemotherapy and after three weeks of receiving 1 st adjuvant cycle of 5-Flurouracil, Epirubicin, Cyclophosphamide (FEC) / Adriamycin (Doxorubicin), Cyclophosphamide (AC)/ Paclitaxel chemotherapy. The serum was separated and stored at -80 ?C until analysis. To avoid false positive results, care was taken to exclude subjects suffering from infectious diseases, allergic diseases, hepatic disorders, cardiac disorders, autoimmune diseases, systemic diseases such as diabetes and other malignancies. The required chemicals- Reduced glutathione, 1- chloro-2, 4-dinitrobenzene (CDNB) and 5-5’- Dithiobis, 2-nitrobenzoic acid (DTNB) were purchased from Alfa Aesar, South Korea. Measurement of Reduced glutathione (GSH) GSH content of serum was determined by the method of Moron et al 20. GSH was determined by the use of standard curve and was expressed as mg/dl. Measurement of Glutathione-s-transferase (GST) Serum GST was estimated by CDNB method 21 . GST values were expressed as IU/L. Measurement of Nitric oxide (NO• ) Serum NO• level was determined in terms of nitrate and nitrite by kinetic cadmium reduction spectrophotometric method 22 with detection limits in serum of 2-250 µmol/L and CVs of 9% and 4.7% for nitrate concentrations of 31.4 µmol/L and 80.2 µmol/L respectively. NO• values were expressed as µmol/L. Statistical analysis The data for biochemical analysis was expressed as Mean ± SD. The statistical significance of the results was analyzed by using one-way ANOVA and Student’s t test. Values of P<0.05 were considered significant. Bivariate correlation was used to analyze the relation between these parameters before and after chemotherapy.
RESULTS
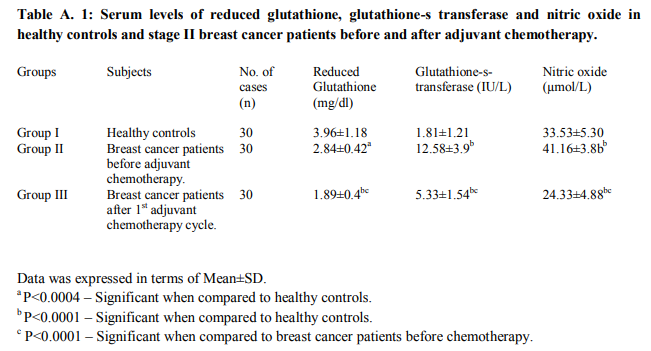
Table A.1 shows the mean serum levels of reduced glutathione, glutathione-s-transferase and nitric oxide in healthy controls and stage II breast cancer patients before and after adjuvant chemotherapy. The serum levels of reduced glutathione were significantly lower in postoperative breast cancer patients before chemotherapy (group II) as compare to healthy controls (group I) (P<0.0004). Further significant decrease in the levels was observed in breast cancer patients after 3 weeks of receiving 1st cycle of adjuvant chemotherapy (group III) as compare to levels before chemotherapy (group II) (P<0.0001) as well as compare to healthy controls (group I) (P<0.0001). The serum levels of enzyme glutathione-s-transferase were found significantly higher in post-operative breast cancer patients before chemotherapy (group II) as compare to healthy controls (group I) (P<0.0001). Serum GST levels were decreased significantly in breast cancer patients after 3 weeks of receiving 1 st adjuvant chemotherapy cycle (group III) as compare to levels before chemotherapy (group II) (P<0.0001) but the levels were still significantly higher as compare to healthy controls (group I) (P<0.0001). The serum levels of nitric oxide were found significantly higher in post-operative breast cancer patients before chemotherapy (group II) as compare to healthy controls (group I) (P<0.0001). But the levels were decreased significantly in breast cancer patients after 3 weeks of receiving 1 st adjuvant chemotherapy (group III) as compare to levels before chemotherapy (group II) (P<0.0001) and as compare to levels in healthy controls (group I) (P<0.0001). Using bivariate correlation analysis of the measured parameters, we found a significantly inverse correlation between GSH and NO• (r= -0.95) before chemotherapy and significant positive correlation between serum levels of GSH and NO• (r=+0.96) and GSH and GST (r=+0.91) after chemotherapy.
DISCUSSION
In the present study, to assess the effect of adjuvant chemotherapy on serum levels of GSH, GST and NO• we have investigated serum levels of these parameters post-operatively in stage II breast cancer patients before start of chemotherapy and after 3 weeks of receiving 1st cycle of adjuvant chemotherapy. Adjuvant chemotherapy is widely used systemic treatment for breast cancer patients after surgical removal of tumor 23. Many reports have demonstrated that the anticancer drugs of different classes are known to generate a high level of oxidative stress in biological systems 7, 24, 25 . Free radical generation is controlled by antioxidant systems that act as protection against free radicals 2 . The glutathione and glutathione dependent enzymes can directly scavenge free radicals and protects cells from oxidative insults 3 . Reduced glutathione also acts as substrate for GST during detoxification of electrophilic compounds 3 . In the present study, we have observed significantly lower levels of GSH in post-operative breast cancer patients before undergoing adjuvant chemotherapy as compare to healthy controls (P<0.0004). The decreased levels of GSH after surgery have been reported in patients with gastric cancer 26. However, in contrast to our finding, elevated levels of GSH in breast cancer patients 1, 27 and non-significant decrease in the levels after 3 weeks of mastectomy have also been reported 27. We found a significant inverse correlation between levels of GSH and nitric oxide in post-operative stage II breast cancer patients before chemotherapy (r= - 0.95). The lower levels of GSH after surgery and before chemotherapy may be associated with higher levels of NO• before chemotherapy that causes increase in formation of peroxynitrite and further oxidation of GSH 15 or it may be possible that GSH could be utilized in the formation of GSNO to neutralize radical NO• . GSH may also be utilized in the detoxification of lipid hydroperoxides that are formed because of high oxidative and nitrosative stress 25. After 3 weeks of administration of 1st adjuvant chemotherapy cycle, we found further significant decrease in serum levels of GSH as compare to levels before chemotherapy (P<0.0001) and as compare to levels in healthy controls (P<0.0001). This is in agreement with the previous study reports 7, 28 . The metabolism of antineoplastic drugs produces highly reactive electrophiles and oxidative stress. Thus produced oxidative stress by antineoplastic drugs is counterbalanced by various antioxidants of the body. GSH is one of the major antioxidant and this high oxidative stress might result in reduction in its levels after chemotherapy 25 . Glutathione-s-transferase catalyses conjugation of GSH with highly reactive electrophiles and thereby plays an important role in detoxification of chemotherapeutic drugs 25. High concentrations of GST may rapidly detoxify anticancer agents and thus prevents their cytotoxic action 1 . In this study, we found significantly higher values of GST in stage II of breast cancer patients post-operatively before chemotherapy as compare to healthy controls (P<0.0001). This is in agreement with previous study reports 29, 30. The level of GST in serum is found to be elevated in most of the human cancers studied and is induced upon oxidative stress 6, 21. The higher values were observed postoperatively because normalization of GST levels after surgery may take about a month 30 . Further, we found significant decrease in the serum levels of GST after 3 weeks of receiving 1st cycle of adjuvant chemotherapy as compare to levels before chemotherapy (P<0.0001) but the values were still significantly higher as compare to healthy controls (P<0.0001). Similar findings were reported by Chakraborty et al 25. We found a significant positive correlation between serum levels of GSH and GST after 3 weeks of receiving 1st adjuvant chemotherapy cycle (r= +0.91). The decrease in the GST activity may be associated with GST catalyzed conjugation of GSH with highly reactive electrophiles and thereby playing a major role in detoxification of chemotherapeutic drugs 25. The decrease in GST activity may be related with decrease in GSH after chemotherapy. Nitric oxide is short-lived highly reactive free radical that is involved in multistep process of carcinogenesis 19, 25. NO• reacts spontaneously with available superoxide radical to form the more potent and versatile oxidant peroxynitrite. Peroxynitrite can damage DNA, initiate membrane lipid peroxidation, deplete antioxidant enzyme activity and reduce GSH content 25. In the present study, we found significantly higher levels of serum NO• in post-operative breast cancer patients before undergoing adjuvant chemotherapy cycles as compare to healthy controls (P<0.0001). Some investigators have reported elevated level of nitrate and nitrite at operable stage in serum samples of patients with breast cancer 2, 16, 17, 31. Konukoglu et al. 19 have shown that serum levels of NO• increased in patients with primary breast tumor compared to controls and the values remained at high levels in post-operative breast cancer patients. Our finding is in agreement with report of Konukoglu et al. 19 . It is possible that observed higher values of NO• could be due to secretion of NO• by non-immune cell types or by tumor infiltrating inflammatory cells in response to residual disease. After 3 weeks of administration of 1st cycle of adjuvant chemotherapy, we observed significant decrease in the levels of NO• as compare to levels before chemotherapy (P<0.0001) and as compare to levels in healthy controls (P<0.0001). We found a significant positive correlation between GSH and NO• after chemotherapy (r=+0.96). The observed decrease in the levels of NO• after chemotherapy may be associated with decreased levels of GSH as adequate GSH level is required for the regulation of iNOS activity by NF-κB activation and optimal synthesis of NO• 11, 12, 13. The other possible mechanism for decreased levels of NO• could be its utilization in the formation of GSNO adduct with GSH (11) .
CONCLUSION
In conclusion, the findings of the study suggests that administration of chemotherapeutic drugs in adjuvant setting in breast cancer patients causes increase in oxidative stress as indicated by decreased levels of antioxidant glutathione. The observed decrease in levels of GST and NO• might be associated with decrease in levels of glutathione in these patients after chemotherapy. However, the present study is a preliminary report and more research with large study group is needed to confirm the findings. Further studies are in progress to evaluate the levels of these parameters and their relationship with apoptosis markers in different stages breast cancer patients receiving chemotherapy in adjuvant setting.
ACKNOWLEDGEMENT
Authors acknowledge the great help received from the scholars whose articles cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed. Authors are grateful to IJCRR editorial board members and IJCRR team of reviewers who have helped to bring quality to this manuscript.
References:
1. Rajneesh CP, Manimaran A, Sasikala KR, Adaikappan P. Lipid peroxidation and antioxidant status in patients with breast cancer. Singapore Med J 2008; 49(8):640-3.
2. Gonec A, Erten D, Aslan S, Akinci M, Simsek B, Torun M. Lipid peroxidation and antioxidant status in blood and tissue of malignant breast tumor and benign breast disease. Cell Biol Int 2006; 30:376-80.
3. Prabasheela B, Singh AK, Fathima A, Pragulbh K, Deka NJ, Kumar R. Association between antioxidant enzymes and breast cancer. Recent Res Sci Technol 2011;3(11): 93-5.
4. Simone CB, Simone NL, Simone V, Simone CB. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill survival, Part 1. Altern Ther Health Med 2007; 13(1):22-8.
5. Horn SL, Fentiman IS. The role of nonsteroidal anti-inflammatory drugs in the chemoprevention of breast cancer. Pharmaceuticals 2010;3:1550-60.
6. Toyokuni S, Okamato K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Letters 1995; 358:1-3.
7. Panis C, Herrere AC, Victorino VJ, Campos FC, Freitas LF, De Rossi T, et al. Oxidative stress and hematological profiles of advanced breast cancer patients subjected to paclitaxel or doxorubicin chemotherapy. Breast Cancer Res Treat 2012;133:89-97.
8. Ewadh MJ, Kadhum NH, Al Hamdani KJ, Alawad AS. Relationship between antioxidants glutathione and total α-L-fucose as tumor markers in breast cancer patients. Medical Journal of Babylon 2009;6(1):164- 75.
9. Sivakumar S, Devaraj N. Enzymatic and nonenzymatic antioxidant status of breast cancer patients in Tamilnadu. IJPBS 2011;2(4):B46- 53.
10. Davis W, Ronai Z, Tew KD. Cellular thiols and reactive oxygen species in drug-induced apoptosis. J Pharmacol Exp Ther 2001;296:1- 6.
11. Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr 2004;134:489- 92. `
12. Harbrecht BG, Silvio MD, Chough V, Kim YM, Simmons RL, Billiar TR. Glutathione regulates nitric oxide synthase in cultured hepatocytes. Ann Surg 1997; 225(1):76-87.
13. Vos TA, Goor HV, Tuyt L, De JagerKrikken, A,Leuvenink R, Kuipers F, et al. Expression of inducible nitric oxide synthase in endotoxemic rat hepatocytes is dependent on the cellular glutathione status. Hepatology 1999;29:421-26.
14. Bakan E, Taysi S, Polat MF, Dalga S, Umudem Z, Bakan N, et al. Nitric oxide levels and lipid peroxidation in plasma of patients with gastric cancer. Jpn J Clin Oncol 2002;32(5):162-6.
15. Mahdy EM, Shousha WG, Ahmed HH, Metwally FM, Ramadan SS. Significance of serum HGF, Bcl-2 and Nitric oxide in primary breast cancer. Nature and Science 2011;9(5):34-41.
16. Coskun U, Gunel N, Sancak B, Onuk E, Bayram M, Cihan A. Effect of tamoxifen on serum IL-18, vascular endothelial growth factor and nitric activities in breast carcinoma patients. Clin Exp Immunol 2004;137:546- 51.
17. Alagol H, Erdem E, Sancak B, Turkmen G, Camlibel M, Bugdayci G. Nitric oxide biosynthesis and malondialdehyde levels in advanced breast cancer. Aust N Z J Surg 1999;69 :647-50.
18. Ewadh MJ, Kadhum NH, Al Hamdani KJ, Alawad AS. The relation between antioxidants Glutathione, Glutathione-stransferase as tumor markers in breast cancer patients. Medical Journal of Babylon 2009;6(1):36-44.
19. Konukoglu D, Turhan MS, Celik V, Turna H. Relation of serum vascular endothelial growth factor as an angiogenesis biomarker with nitric oxide and urokinase-type plasminogen activator in breast cancer patients. Indian J Med Res 2007;125:747-51.
20. Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione-s-transferase activities in rat lung and liver. Biochim Biophys Acta 1979;582:67-78.
21. Prabhu K, Bhat GP. Serum total glutathiones-transferase levels in oral cancer. J Can Res Ther 2007;3:167-8.
22. Cortas NK, Wakid NB. Determination of inorganic nitrate in serum and urine by a kinetic cadmium reduction method. Clin Chem 1990;36:1440-3.
23. Peters WHM, Roelofs HMJ, Putten WLJV, Jansen JBMJ. Response to adjuvant chemotherapy in primary breast cancer: no correlation with expression of glutathione-stransferases. Br J Cancer 1993;68:86-92.
24. Conklin KA. Cancer chemotherapy and antioxidants. J Nutr 2004;134(11):3201-4S.
25. Chakraborty P, Ugir HSK, Murmu N, Das JK, Pal S, Bhattacharya S. Modulation of Cyclophosphamide –induced cellular toxicity by diphenylmethyl selenocyanate in vivo, an enzymatic study, J Cancer Molecules 2009;4(6):183-9.
26. Czeczot H, Scibior D, Skrzycki M, Podsiad M, Porembska Z. Glutathione level and activity of GSH dependent enzymes in gastric carcinoma patients-a preliminary report. Gastroenterol Pol 2005;12(2):107-11.
27. Mishra S, Sharma DC, Sharma P. Studies of biochemical parameters in breast cancer with and without metastasis. Indian J Clin Biochem 2004;19(1):71-5.
28. Kasapovic J, Pejic S, Stojiljkovic V, Todorovic A, Radosevic-Jelic L, Saicic ZS, et al. Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages after chemotherapy with 5-fluorouracil, doxorubicin and Cyclophosphamide. Clin Biochem 2010;43(16-17):1287-1293.
29. Prabasheela B, Baskaran S, Alteration of glutathione dependent enzymes in pre and post-operative breast carcinoma, JBMAS. 1- 7.
30. Severini G. Glutathione-s-transferase activity in patients with cancer of the digestive tract. J Cancer Res Clin Oncol 1993;120(1-2):112-4.
31. Gunel N, Coskun U, Sancak B, Hasdemir O, et al, Prognostic value of serum IL-18, and nitric oxide activity in breast cancer patients at operable stage. Am J Clin Oncol 2003; 26(4):416-21.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License