IJCRR - 9(5), March, 2017
Pages: 41-48
Date of Publication: 20-Mar-2017
Print Article
Download XML Download PDF
Prevalence of asymptomatic malaria parasitaemia during pregnancy and its effect on foetal birth weight
Author: Owa OO1, 2, Eniowo AR2, Adedosu AN3, Ogunro PS4, Faturoti SO2, Ogunro AA2
Category: Healthcare
Abstract:Background: Pregnant women in endemic area may experience malaria infection without clinical symptoms. Its effects on the neonatal outcomes mayalso occur in this asymptomatic state.
Objective: To determine the prevalence of asymptomatic malaria parasitaemia in pregnancy and the relationship between the level of malaria parasitaemia and foetal birth weight.
Materials And Methods: A total of 290 asymptomatic paturients and their babies were recruited over 4 months with informed consent. The maternal, placenta and cord blood samples were obtained and examined for level of malaria parasitaemia. New-borns were weighed and classified as normal birth weight (?2500 g) or LBW (< 2500 g).Pearson correlation was used to determine the relationship between the levels of malaria parasitaemia and birth weights. Student's t and Pearson chi-square tests were used to compare means and percentages.
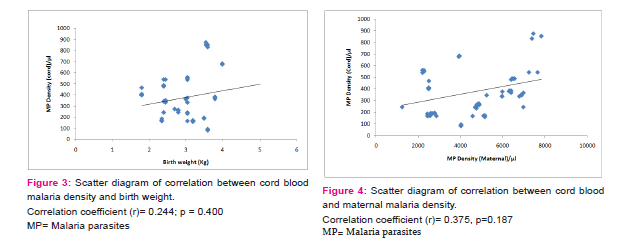
Results: The prevalence of malaria parasitaemia was 32.8%, 31% and 24.1% in the maternal, placental and cord blood smear respectively. The prevalence of low birth weight was 12.1% with women with malaria delivered more LBW babies (31.6%) than their uninfected counterparts (2.6%) (p=0.006). However, correlation showed a weak positive correlation between the levels of maternal parasitaemia(r = 0.163; p=0.504) and cord blood parasitaemia(r = 0.244; p = 0.400) but weak negative correlation with the level of placental parasitaemia (r = 0.135; p= 0.598) and foetal birth weight which were not statistically significant.
Conclusion: There was no significant correlation between the level of malaria parasitaemia and birth weight in asymptomatic parturients. However, the main impact on pregnancy outcome was the higher prevalence of LBW.
Keywords: Malaria parasitaemia, Pregnancy, Foetal birth weight
Full Text:
INTRODUCTION
Malaria remains a major health concern worldwide; perhaps the most important parasitic infection affecting mankind with an estimated 3.3 billion people at risk of malaria in 2010 worldwide.1 An estimated 655,000 deaths were recorded globally in 2010 of which 86% were children less than 5 years of age. The disparity in region specific mortality is huge with 91% of all deaths recorded by WHO in Africa region.1 Women are more susceptible to malaria during pregnancy and in the puerperium.2, 3
In areas where malaria is endemic, that is stable malaria transmission like Nigeria, at least one in four pregnant women has evidence of peripheral or placental malaria at delivery.4, 5 However, most cases of malaria in pregnancy in such areas may remain asymptomatic thus undetected and untreated.6 The pre-existing immunity retained during previous exposures protect against clinical malaria. Unfortunately, this subclinical infection poses great challenge to mother and foetus.4, 7
The mechanism underlying increased susceptibility to malaria and the severity of the disease in pregnancy is not fully understood.8,9 It has been suggested that despite the acquired antimalaria immunity of these pregnant women, the uteroplacental vascular space apparently provides a site for parasite sequestration and development.10,11
The main effects of malaria in pregnancy on birth outcomes are thought to be mediated by maternal anaemia12,13,14 and placental insufficiency.11,15 Both factors have been suggested to act together to cause either intrauterine growth restriction (IUGR) or preterm delivery leading to low birth weight (<2,500g).16,17,18 Malaria is one of the causes of severe anemia in pregnancy.19 Over 26% of anaemia in pregnancy is attributed to malaria, and malaria related maternal deaths are reaching an unacceptable rate of 23%.5,20
Low birth weight (<2,500g) is known to be an important risk factor for infant mortality, an important cause of foetal and neonatal morbidity and one important determinant of infant healthy growth and development.4,13,21 Up to 20% of LBW in sub-Saharan Africa has been attributed to malaria in pregnancy and this figure represents 35% of preventable low birth weight in the region.8, 12
The prevalence of asymptomatic malaria parasitaenia in pregnancy and its effect varies even within the endemic region as reported by various workers.21,22,23 In Nigeria, few studies focusing on malaria in the peripartum period have been conducted but with variable findings.23,24,25
Malaria in pregnancy still remains a major health concern in the Sub-Saharan Africa. Malaria is thought to be an important contributor to the 3.5 million LBW babies born annually in sub-saharan Africa,8 attributable fraction is estimated to be 19%.8,23 Most of the studies done focusing on peripartum malaria parasitaemia in Nigeria did not distinguish the effect of an asymptomatic parturient, thus the importance of asymptomatic carrier is poorly elucidated. Therefore, this present study was designed to study prevalence of asymptomatic peripartum malaria parasitaemia and its effects on birth weight.
MATERIALS AND METHOD
The study took place at the Federal Medical Centre, Owo is a tertiary health centre, located Owo, Ondo State, South West Nigeria. Ondo State is located entirely within the tropics with annual rainfall between 150mm and 2,000mm.27,28 Malaria transmission in Southwest Nigeria is perennial but seasonal and peaks during the rainy season, which normally runs from April to November. The study received ethical approval from Federal Medical Centre, Hospitals Management Board ethics review committees.
The study was a prospective study using a purposive non-probability random sampling method, there was recruitment of all consenting parturient in labour that satisfied the inclusion criteria until the desired sample size is completed. Their babies were weighed at birth, samples obtained and the proforma filled. Inclusion criteria were confirmation of active phase of labour; asymptomatic, non-febrile parturient and singleton pregnancy without any known congenital anomaly. Exclusion from the study are subjects that refused to participate in the study; those on anti-malaria at the time of labour; presence of any medical illness such as diabetes mellitus, chronic renal disease, haemoglobinopathies, HIV/AIDs, chronic hypertension and severe pre-eclampsia/ eclampsia and presence of multiple gestation, intrauterine foetal death and obvious foetal anomalies. There were 290 women recruited having met the inclusion criteria.
Sample collection
In labour, 5mls of blood sample was collected from the mother, 3mls of blood was collected from the clamped cord immediately after delivery and placenta blood was also obtained into EDTA bottles. The placental weight, birth weight and pregnancy outcome was recorded. In this study, malaria infection was taken as the presence of asexual P. falciparum parasites of any density, in a thick film.29,30
Analytical methods
Hemoglobin (Hb) was measured using the method of Schoen and Solomon17 and determination of packed cell volume (PCV) by the microhematocrit method. Malaria parasitaemia was estimated in thick and thin film from maternal blood, cord blood and placental aspirate of all samples collected, according to the method described by Cheesbrough.31 Peripheral blood, parasite density was determined by counting asexual forms of the parasite per 100 white blood cells (WBC) converted to parasites/µL using a predetermined blood sample total white blood cell count.31,32 Parasite density was graded as Low (parasites < 1,000/µL), Moderate (1,000- 4,999/µL) and high (>5,000/µL).33 Quality Assurance was maintained by randomly selecting 5 slides in a pool of every 30 slides for comparison in the institution’s main laboratory. The babies were weighed within 30 minutes of birth after drying the body using a bassinet weighing scale (Salter model 180 made in England 2002-0218015B) with maximum 15kg capacity and scale precision of 50g
Statistical Analysis
Data was analysed using the (SPSS) Version 17 (SPSS Inc, Chicago, IL). Analysis included the use of descriptive statistics such as means and standard deviation of age, parity and haemoglobin concentration. Using student t-test and chi square test for association between maternal and babies’ characteristic and malaria parasitaemia were determined. P-value of ≤ 0.05 was taken as significant. Multivariate analysis using linear regression of maternal age, parity, previous antimalaria use and maternal parasitaemia was done. Expression of percentages, frequency tables and charts was used to illustrate the presence of parasitaemia (maternal, cord and placenta) and risk factors for LBW. Pearson correlation was used to determine the relationship between the levels of parasitaemia and birth weights, these were shown on a scatter plots.
RESULTS
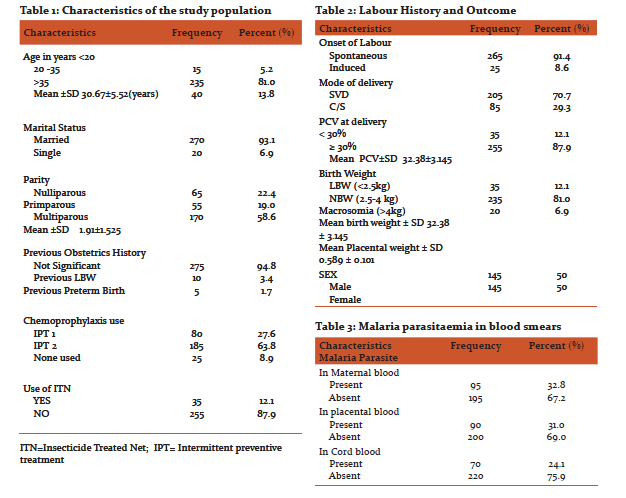
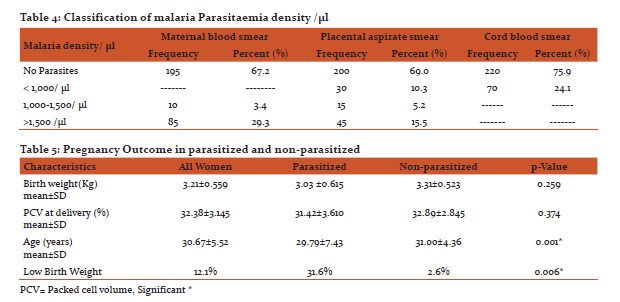
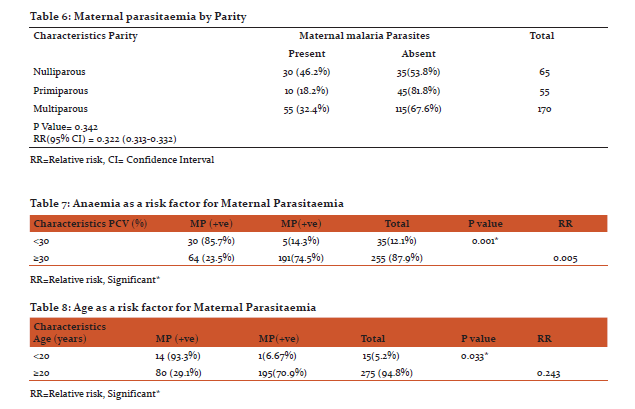
Sociodemographic background of the study population is shown in Table 1; with 290 women who met the inclusion criteria were recruited for the study. Their age ranged from 18- 44 years with mean age of 30.69±5.52 and modal age of 33. The mean packed cell volume (PCV) at delivery was 32.38±3.145% and ranged from 26 to 40%. All babies were delivered alive with no still birth in this study. Mode of delivery; 70.7% of the labour had spontaneous vaginal deliveries with 29.3% ended up in a caesarean delivery as shown in Table 2. There were 95 women (32.8%) with positive maternal parasitaemia, 90 positive placental smears (31.0%) while positive cord blood parasitaemia were 70 (24.1%) as shown in Table 3. The cumulative prevalence of malaria infection at delivery (total number of women positive for malaria either by peripheral, cord blood or placental blood smear examination) was 29.3% (255/870). When the positive maternal parasitaemia was related to parity, the number of positive smears in nulliparous (30) 46.2%, primiparous (10) 18.2% and multiparous (55) 32.4% however, the differences were not statistically significant (P =0.346). The level of malaria parasitaemia is higher in the maternal peripheral blood smear compared with placental and cord blood smears as shown in table 3. The same difference was noted with the parasite density as shown in Table 4. The mean parasite density in maternal blood was 4,308±1896.67/µl, in placental smear was 1,853±1351.36/µl and in cord blood was 368±217.49/µl. Of the 290 cord samples collected, 70 (24.1%) were found to be positive for asexual forms of plasmodium falciparum, thus giving a prevalence of congenital malaria to be 24.1%. However, overall parasite density in cord blood is lower than both peripheral and placental parasitaemia density as show in Table 4.
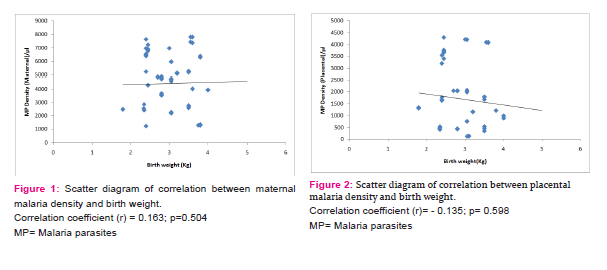
Parasitaemia was associated with lower mean birth weight 3.03k g ±(0.615) versus 3.31k g ±(0.523) among mothers with peripheral parasitaemia; P = 0.257 as described in Table 5. Low birth weight (LBW) occurred in 35/290 deliveries thus giving a prevalence of 12.1%. Peripheral parasitaemia was also significantly associated with LBW babies (30/95 [31.6%] versus 97/195 [2.6%]; P =0 .006, OR = 1.6 [1.1–2.5]). Preterm deliveries contributed 14.3% of the LBW babies. Figure 1, 2 and 3 shows the correlation between the birth weight and maternal, placental and cord blood parasitaemia respectively. The correlation coefficient were; maternal=+0.163, p=0.504, placental= -0.135, p=0.594 and cord blood=+0.244, p=0.400. There was a positive correlation between parasite densities in the peripheral film and cord blood film. The correlation coefficient was +0.375, p=0.187. See Scatter plot illustration in figure 4.
DISCUSSION
Malaria in pregnancy contributes significantly to maternal and perinatal morbidity and mortality in sub Saharan African.6, 8,26,34 This study illustrates the continuing impact of malaria in otherwise healthy asymptomatic pregnant Nigerian women, 32.8% of whom had peripartum malaria parasitaemia. This rate confirm recent reports in pregnant or at parturition in Nigerian women 6, 35, 36 although prevalence ranging from 12.5 to 80% 23, 35, 37 had been reported in Nigeria. The findings is also in agreement with findings from Cameroon, Senegal and Sierra Leone38, 39, 40 but higher than in northern Nigeria.26 Several factors could have accounted for the disparity in the prevalence like the season, study population characteristics (socio-economic class, parity, and age), the training of the microscopist, the use of chemoprophylaxis (IPT, ITN) and the study design. In this study, parasitaemia at the time of delivery was found to be associated with nulliparity and maternal age. Several other studies have reported similar associations.6,41 The mean age of parasitized parturients in this study was significantly lower than the unparasitized parturients (29.79±7.43 was compared to 31±4.36) years (p = 0.001) as shown Table 5, similar observation was made by other investigators.6,41 This suggested that pregnancy-associated immunity and naturally acquired immunity increases with age. The hypothesis was that the development of pregnancy associated immunity, for example, production of antibodies that inhibit adherence of placental parasites to chondroitin sulphate A, may be very important in women <25 years of age who have lower levels of acquired immunity. While older women living in such endemic areas due to repeated exposures, may have obtained adequate immunity and are thus less dependent on anticytoadherent antibodies. Parasitaemia in the mothers was found to be associated with lower maternal packed cell volume. This finding is not unusual as the association of malaria in pregnancy and low hematocrit has been recognized and reported by previous workers.6 The drop in hematocrit occurs as a result of the fact that parasitized and unparasitized erythrocytes are destroyed by the spleen during malaria infection. It is however known that using of antimalaria drugs that are normally effective within a locality significantly reduces the occurrence of this anaemia.42 Although the mean PCV of those parturient that had malaria parasitaemia was lower than those without parasitaemia (31.42% ±3.61 compared to 32.89%±2.85) the difference between the means was not statistically significant (p= 0.374) Table 5. This may be due to the fact that majority of the patients in this study used malaria chemoprophylaxis.
The main effect of maternal parasitaemia on the babies was the reduction in the birth weight. This is consistent with the observations from other malaria endemic countries.6,38, 43 The impact of malaria during pregnancy on LBW in sub-Sahara Africa has been extensively reviewed.8 The LBW prevalence in this study was 12.1% and significantly increased with mothers with parasitaemia. This is within 8-25% range reported from previous studies from Sub-saharan Africa.24, 44, 45
Multivariate analysis showed that younger maternal age less than 20years, booking status, use of chemoprophylaxis and PCV were significantly associated with malaria parasitaemia in the women. Younger maternal age and hematocrit level were also described by other investiators.6,26,39 These authors observed that younger but not older primigavidae were more likely to have placental malaria. This may explain the weak inverse correlation observed in this study with placental parastaemia and birth weight.
There was a positive correlation between parasite densities in the peripheral film and cord blood film. This implies that a population with increased prevalence asymptomatic maternal parasitaemia at delivery may have high prevalence of congenital malaria.
Chemoprophylaxis during pregnancy has been shown to reduce the risk of malaria infection in pregnant women significantly.26 This was corroborated in this study with a significant association between chemoprophylaxis and reduction of malaria parasitaemia in cord blood (P= 0.05, RR= 0.0813) but reduction was not statistically significant for maternal and placental blood smears. This finding may be due to the fact that out of 91.4% (265 out of 290) that used chemoprophylaxis only 63.8% (185 out of 265) received the two doses of sulphadoxine pyrimethamine (SP) in contrast to high rate reported by some workers.26 The two doses of IPT had been proven to be the currently most effective chemoprophylaxis for prevention of malaria during pregnancy in areas where transmission of Plasmodium falciparum malaria is stable like ours.1, 26 The observed impact of malaria on the mother and their newborns add justification for promoting use of malaria preventive measure in pregnancy.
CONCLUSION
In Nigeria, one in every four asymptomatic women has malaria (maternal, placental and/ or cord) parasitaemia at delivery. Maternal age less than 20 years was the most important predisposing factor. There was no significant correlation between the level of malaria parasitaemia and birth weight in asymptomatic parturients. However, the main impact on pregnancy outcome was the higher prevalence of LBW.
ACKNOWLEDGEMENTS
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
Source of funding: None
Conflicts of interest: Non
References:
1. World Health Organization. World Malaria report 2011, Geneva. World Health Organization: 2011 p 200 .
2. Diagne N, Rogier C, Sokhna CS, Tall A, Frontenille D, Roussilhon C, et al. Increased Susceptibility to Malaria during early Postpartum Period. N. Engl J. Med. 2000; 343(9): 598-603.
3. Terkuile FO, Terlouw DJ, Phillips-Howard PA, Hawley WA, Friedman JF, Kariuki SK., et al. Reduction of Malaria during Pregnancy by Permethrin - treated bed nets in an area of intense Perennial Malaria Transmission in Western Kenya. Am. J. Trop. Med. Hyg. 2003; 68 (4 suppl.): 50-60.
4. Oraneli BU, Okeke OC, Ubachukwu PO. Effect of placental malaria on birth weight of babies in Nnewi Anambra State. Nigeria. J. Vect. Borne Dis. 2013; 50(1):13-17.
5. Houmsou RS, Amuta EU, Sarr TT, Adie AA. Malaria infection in pregnant women attending antenatal clinics in Gboko Benue State, Nigeria. Int. J. Acad. Res 2010; 2:33-36.
6. Mokuolu OA, Falade CO, Orogade AA, Okafor HU, Adedoyin OT, Oguonu TA, et al. Malaria at Parturition in Nigeria: Current Status and Delivery Outcome. Infect Dis in Obstet Gynecol 2009; 2009: 473971.
7 Steketee RW, Wirima JJ, Slutsker L, Heymann DL, Breman JG. The problem of malaria and malaria control in pregnancy in Sub Saharan Africa. Am J Trop Med Hyg 1996; 55: 2-7.
8. Guyath HL, Snow RW. Impact of Pregnancy on Low Birth Weight in Sub-Saharan Africa. Clin. Microbiol. Rev. 2004;17 (4): 760-769.
9. Guyatt HL, Snow RW. Malaria in Pregnancy as an indirect cause of Infant Mortality in Sub-Saharan Africa. Trans R. Soc. Trop. Med. Hyg. 2001; 95: 569-576.
10. Fried M, Duffy PE. Adherence of Plasmodium Falciparum to ChondrotinSulphate A in the human placental.Science 1996; 272:1502-4.
11. Rogerson SJ. Hviid L. Duffy PE. Malaria in Pregnancy Pathogenesis and Immunity. Lancet Infec Dis 2007; 7(2): 105-117.
12. Uneke CJ. Impact of Placental Plasmodium Falciparium Malaria on Pregnancy and Prenatal Outcome in sub-Saharan Africa. Introduction to Placental Malaria Yale J. Biolo. Med. 2007; 80(2): 39-50. II. Effect of Placental Malaria on Perinatal Malaria and HIV pp 95-103.
13. Brabin B, Piper C. Anaemia –and malaria attributable low birth weight in two populations in Papua New Guinea. Ann Hum Biol 1997; 24:547-555.
14. Kasumba IN, Nalunkuma AJ, Mujuzi G. Kataka FS, Byaruhanga R, Okong P, Egwang TG. Low birth weight associated with maternal anaemia and plasmodium falciparum infection during pregnancy in a peri-urban/urban area of low endemicity in Uganda. Ann. Trop. Med. Parasitol. 2000; 94:7-13.
15. Beenson JG, Amin N, Kanjala M, Rogerson SJ. Selective accumulation of mature asexual stages of plasmodium falciparum –infected erythrocytes in the placenta. Infect Immun 2002; 70:5412-5415.
16. Moormann AM, sullivan AD, Rochford RA, Chensue SW, Bock PJ, Nyirenda T, Meshnick SR. Malaria and Pregnancy: placental Cytokine expression and its relationship to intrauterine growth retardation J. infect Dis 1999; 180: 1987-1993.
17 Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kehigwa E, Font F, Alonso PL. The impact of placental malaria on gestational age and birth weight. J Infect Dis 2000, 181:1740-1745.
18.. Suguitan AL. Jr, Cadigan TJ, Nguyen TA, Zhou A, Leke RJ, Metenow S, Thuita L, Megnekou R, Fogako J, Leke RG, Taylor DW. Malaria associated cytokine changes in the placenta of women with pre-term deliveries in Yaoundé, Cameroon. Am. J. Trop. Med. Hyg. 2003, 69.574-581.
19. Rijken M, McGready R, Boel ME, Poespoprodjo R, Singh N, Syafruddin D, Rogerson S, Nasten F. (2012) Malaria in Pregnancy in the Asia- Pacific Region. Lancet Infect Dis. 12: 75-88.
20 Nduka FO., Eybu A., Okafor C., Nwaugo CO. Prevalence of malaria parasites and anaemia in pregnant and non-pregnant women in Aba and Okigwe towns of South East Nigeria. Animal Research International 2006; 3(3): 506-512.
21. Akum AE, Kuoh AJ, Minang JT, Boyo MA, Mokube JA, Troye-Blomberg M. The effect of maternal, unbilical cord and placental malaria parasitaemia on the birthweight of newborns from south-western Cameroon. Actra Paediatr(Stockholm) 2005; 94:917-23.
22. Ogunsedun A, Kofie BAK., Adetunji JA, Fakoya EAO, Bamgboye EA. Prevalence and Significance of Asymptomatic Malaria Parasitaemia in Sagamu, Nigeria.Nig. J. of Parasitology 1990; 9:145-158.
23. Sule-Odu OA, Ogunledun A, Olatunji AO. Impact of asymptomatic malaria parasitaemia at parturition on perinatal outcome J. of Obstet Gynaecol 2003; 22(1):25-28.
24. Ibhanesebhor SE and Okolo AA. Placental malaria and pregnancy outcome. Int J GynecolObstet1992;37(4):247–252.
25. Mukhtar MY, Lesi FEA, Iroha EU, Egri-Okwaji MTC, and Mafe AG. Congenital malaria among inborn babies at a tertiary centre in Lagos, Nigeria.J. Trop Paediata 2006;52(1):19-23.
26. Panti AA, Omokanye LO, Ekele BA, Jiya NMA, Isah AY, Nwobodo EI, Ahmed Y. The prevalence of asymptomatic malaria parasitaemia at delivery in Usmanu Danfodiyo University teaching Hospital Sokoto North Western Nigeria. Glo. Res. J. Med 2012; 2(4): 48-53.
27. Bola O. Profile of Ondo State. Coastal News of July 2010 Available at www.coastalnews-com/627-profile of Ondo State
28. Adeyemi A. Ondo State “The sunshine State”. Welcome to Nigeria March 18, 2011;http://wwwcometomgeria.Com/search-by-region/south-west /ondo-state.
29. Sowanmi A, Abohweyere AEJ, Akindele JA. Ilesanmi AO, Folade CO, Oduola AMJ. Comparison of the incision and aspiration Methods for the Diagnosis of Placental Malaria Infection. J of ObstetGynaecol. 1996;16(5): 316-320.
30. Ngassa PC. Malaria Parasitaemia and the risk of preterm Labour. A Re-Evaluation of the Evidence. African Journal of Reproductive Health 2000; 4(2): 53-61.
31. Cheesbrough M. District Laboratary Practice in Tropical Country Part 2 Cambridge University Press United Kingdom 2000 PP 284-312.
32. Trap JF, Rogier C. Combating Malaria Morbidity and Mortality by reducing transmission. Parasitology today. 1996; 12(6):236-240.
33. Greenwood BM and Armstrong JRM. Comparism of two simple methods of determining malaria parasite density. Trans. Royal soc. Trop. Med. Hygiene 1991; 85: 186- 188.
34. Falade CO, Tongo OO, Ogunkunle OO, Orimadegun AE. Effects of malaria in pregnancy on newborn anthropometry. J. Infect DevCtries2010; 4(7):448-453.
35. Anorlu RI, Odum CU, Essien EE. Asymptomatic Malaria Parasitaemia in Pregnancy Women at Booking in a Primary Health Care Facility in a Periurban Community in Lagos, Nigeria. Afr. J. Med and Medical Science. 2001; 30 (Supp): 39-41.
36. Ayoola OO, Whatmore A, Balogun WO, Jarrett OO, Cruickshank JK and Clayton PE. Maternal malaria status and metabolic profiles in pregnancy and in cord blood: relationships with birth size in Nigerian infants. Malar J. 2012; 11:75
37. Obiajunwa PO, Owa JA, and Adeodu OO. Prevalence of congenital malaria in Ile-Ife, Nigeria.Journal of Tropical Pediatrics 2005;51:219-222.
38. N’Dan CT, N’Diaye JL, Gaye A., Le Hesran JY. Placental Malaria and Pregnancy Outcome in a Peri-Urban Area in Senegal Revue d’ Epidemiologieet de SantePublique. 2006; 54(2): 149-156.
39. Tako EA, Zhou A, Lohoue J, Leke R, Taylor DW, and Leke RFG.Risk factors for placental malaria and its effect on pregnancy outcome in Yaounde, Cameroon.Am J. Trop. Med. Hyg.2005; 72(3): 236-242.
40. Morgan HG. Placental Malaria and Low Birth Weight Neonate in Urban Sierra Leone. Ann Trop. Med. Parasitol. 1994; 88:575-580.
41. Kolawole OM, Babatunde AS, Jimoh AAG, Balogun OR, Kanu IG.Risk Determinations to Congenital Malaria in Ilorin Nigeria Asian J. of Microbiol Biotech Env. Sc. 2007; 12(2): 215-222.
42. Garner P, Gulmezoglu AM, “Prevention versus treatment for malaria in pregnant women,” Cochrane Database of Systematic Reviews. 2000; no. 2, Article ID CD000169.
43. Duff PE and Fried M. Malaria in the Pregnant Woman.Current Topics in Microbiology and Immunology.2005; 295: 169-200.
44. Sarr D, Marrama L, Gaye A. High Prevalence of Placental Malaria and Low Brith Weight in SahelianPeri-urborn Area. Am J Trop. Med. Hyg 2006; 75(1): 171-177.
45. Egwunyenga OA, Ajayi JA, Duhlinska-Popova DD, Nmorsi OP. G. Malaria infection of the cord and birth weights in Nigerians. Central Afr J. Med 1996; 42(9): 265-268.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License