IJCRR - 9(11), June, 2017
Pages: 30-35
Date of Publication: 12-Jun-2017
Print Article
Download XML Download PDF
Myocardial Bridges as a Risk Factor for Coronary Atherosclerosis
Author: Lujinovic Almira, Kapur Eldan
Category: Healthcare
Abstract:Introduction: The presence of a myocardial bridge on the ramus interventricularis anterior can lead to myocardial ischemia and changes in the structure of the vessel wall, especially in the segment proximal to the bridge.
Objectives: Examine the wall structure of the ramus interventricularis anterior and estimate the atherosclerosis in its intramyocardial, proximal, and distal segments with an atherosclerosis index.
Materials and Methods: Sections of the ramus interventricularis anterior and its branches were prepared at autopsy from 20 hearts with myocardial bridges. Sections stained with hematoxylin-eosin or elastica van Gieson were examined microscopically, and the thickness of the intima and media was determined with ocularmicrometric measuring to establish an atherosclerosis index (a quotient of intima to media thickness).
Results: In histopathologic analysis, atherosclerotic changes of varying intensity were present in segments proximal to the myocardial bridge. In contrast, no histopathologic evidence of atherosclerosis was present in any intramyocardial segment, and distal segments had only mild atherosclerotic changes. In agreement with the histopathologic findings, the atherosclerosis index value of the proximal segment of the ramus interventricularis anterior was significantly higher than that of the intramyocardial segment and the distal segment of the artery (p < 0.0001), and the highest value was in in the proximal segment, 1.5-2 cm from the entrance into the intramyocardial segment.
Conclusion: The presence of a myocardial bridge in the ramus interventricularis anterior is a risk factor for the development of atherosclerotic changes proximal to the entrance of its intramyocardial segment.
Keywords: Coronary arteries, Myocardial bridges, Atherosclerosis, Histomorphometry
Full Text:
INTRODUCTION
Human coronary arteries and their branches are located in the subepicardial adipose tissue. In some hearts, coronary arteries coursing subepicardially, travel shallowly or deeply into the myocardium to reappear on its surface after an intramyocardial course of variable length [1].
The intramyocardial portion of the coronary artery is named the “tunneled” segment. Bunches of myocardial fibers that pass over the segment and cover it, usually only on one part, shall be designated as myocardial bridge [2]. Reyman was the first, as early as 1737 in autopsy examination, to detect myocardial bridges. In 1960, Portmann and Iwig [3] detected systolic narrowing of the lumen of the ramus interventricularis anterior on coronary angiograms and presumed that the narrowing was caused by contraction of myocardial bridge fibers with consequent compression of the tunnel segment. Scientists who dissected the myocardial bridges found their high frequency (30-60%) and their most frequent location to be in the ramus interventricularis anterior [4-7].
Geiringer [8] reported that atherosclerosis did not develop in the tunnel segment of the coronary descending artery, an observation that prompted attention to the relationship between myocardial bridges and the development and location of atherosclerotic changes. Results of histopathological studies [11-13] have confirmed Geiringer’s opinion that the myocardial bridge protects the tunnel-segment intima from developing atherosclerosis, while favoring the development of intense atherosclerosis proximal to the bridge. The autopsy findings have been supported by in vivo diagnostic methods. Thus, atherosclerotic changes proximal to the tunnel segment were seen in computed tomographic coronary angiograms in about 70% of patients [14-16], but no changes was seen in the wall of the tunnel segment. Also, intravascular ultrasound studies have revealed atherosclerotic changes proximal to myocardial bridges in about 90% of cases 17, 18]. Intravascular ultrasound has been advocated for study of the relationships between myocardial bridges and atherosclerotic changes because it is more sensitive than coronary angiograms in detecting mild atherosclerotic changes [17, 18].
However, not all authors agree there is a relationship between the presence of myocardial bridges and the development or location of atherosclerosis, and claim that atherosclerosis develops about equally often under the myocardial bridge as in other parts of coronary arteries [19-21].
The difference of opinions on the possible influence of myocardial bridges on the occurrence and localization of coronary atherosclerosis has led us to examine the structure of the bridge walls, and to determine, through use of an atherosclerosis index, the degree of atherosclerosis in the intramyocardial segment of the ramus interventricularis anterior as well as proximally and distally to the bridge.
MATERIALS AND METHODS
We used cadaveric hearts from 60 patients (36 male, 24 female; mean age 45.2 ±16.2 years) for this study. When a myocardial bridge was found on dissection, a branch of the ramus interventricularis anterior was sectioned transversely at intervals of 0.5 cm, up to 3 cm proximally and 3 cm distally to the bridge. The sections were fixed in 10% buffered formaldehydeand embedded in paraffin. Paraffin blocks were sectioned at 5 µm thickness and stained with hematoxylin-eosin and elastica van Gieson. Cross-sections of the ramus interventricularis anterior were examined by light microscopy, with careful inspection of the arterial wall for atherosclerotic changes and oculometric measurement of intima and media thickness. The intima was measured at its thickest and thinnest areas, and the mean value was calculated. The atherosclerosis index was expressed as a quotient of intima and media thickness (intima thickness/media thickness). The number of necessary measurements for each segment was determined on the basis of De Hoff’s formula. The total number of measurements for all proximal (P) segments was 162 (40 measurements for each P1 and P2 segment, 20 measurements for each P3 and P4 segment, 18 measurements for P5, and 24 measurements for P6 segments). The number of measurements for the myocardial bridge region, ie, the tunnel segment, was 48. The total number of measurements for distal (D) segments was 99 (20 measurements for each D1, D2 and D3 segments, 17 measurements for D4 segment and 11 measurements for each D5 and D6 segment).
The mean values of the degree of atherosclerosis in the myocardial bridge region and proximal and distal segments were compared with Anova statistical project. The atherosclerosis index in the tunnel segment region and each proximal and distal segment, were also compared with Anova. P value of <0.05 was considered statistically significant.
RESULTS
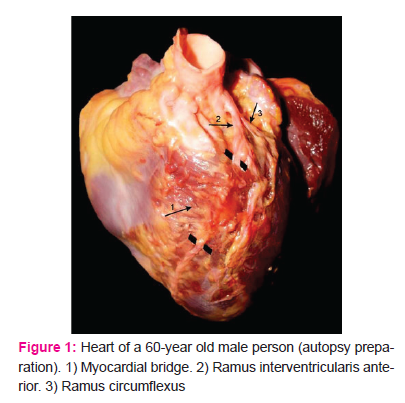
Myocardial bridges in ramus interventricularis anterior arteries were detected in 20 of 60 (33%) hearts examined. The bridge fibers mostly lay transversally to the flow of blood in the vessels (Fig. 1).
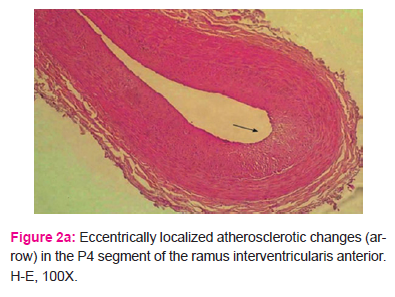
All proximal segments of the ramus interventricularis anterior wall had atherosclerotic changes of diverse intensity, usually with eccentric intimal thickenings of gathered connective fibers, proliferated smooth muscles cells in the intima, and numerous foamy cells (Fig. 2a and 2b).
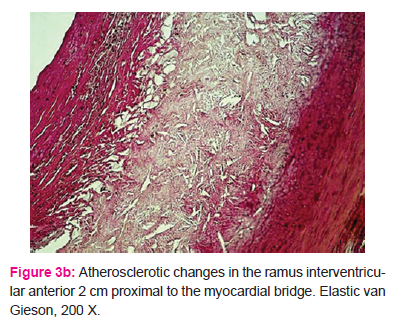
In some cases, atherosclerotic changes were expressed together with the presence of classic fibroatheroma, with an adipose nucleus and fibroid cup on its surface, proximal to the intramyocardial segment (Fig. 3a and 3b).
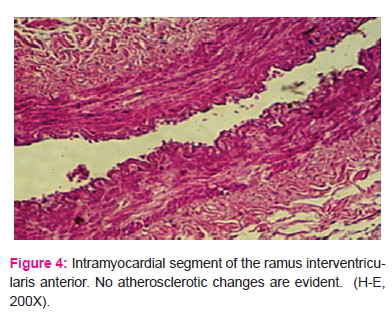
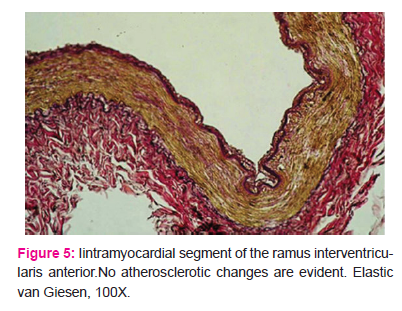
No histopathologic changes indicative of atherosclerosis were present in any case in the intramyocardial tunnel segment (Fig. 4 and 5).
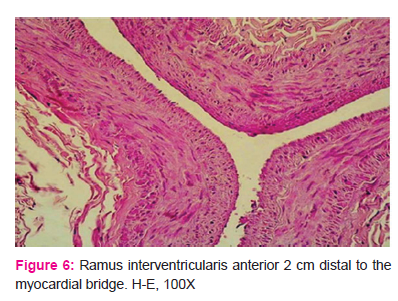
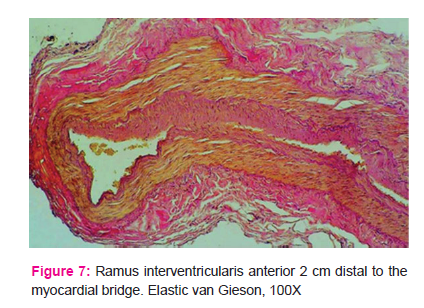
Distal segments of the ramus interventricularis anterior wall had variably weakly to strongly expressed diffuse thickening of the intima, indicative of milder atherosclerotic changes (Fig. 6 and 7).
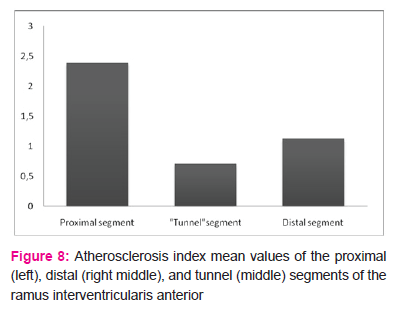
The mean atherosclerosis index value of the intramyocardial segments of the ramus interventricularis anterior was 0.71±0.22. The mean value in the proximal segments (2.39±0.63) was significantly higher than in the distal segments (1.13±0.22) (p<0.001) and in the intramyocardial segment (p<0.001).
Even though values in the distal segments (from exit from tunnel segment exit to 3 cm distant) were lower than those in the proximal segments, they were still significantly higher than in the myocardial bridge region (Fig. 8). The atherosclerosis index mean value of each distal segment (D1-D6) was significantly higher (p<0.001) than the mean value of the same index in the intramyocardial segment .
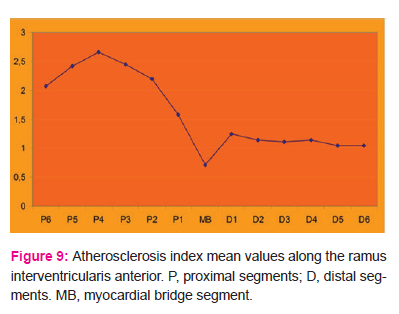
Figure 9 illustrates the atherosclerosis index mean values along the ramus interventricularis anterior. It can be seen that the index was lowest in the intramyocardial segment (0.71), and proceeding proximally from the myocardial bridge, the index rose abruptly and reached its highest value (2.66) in the P4 segment, after which it declined slowly. In the distal segments, the index rose in the first segment to 1.24, then declined to 1.14 in the next segment and remained nearly constant in subsequent segments.
DISCUSSION
The aim of our study was to examine the structure of the intramyocardial segment walls and to determine, with use of an atherosclerosis index, the degree of atherosclerosis in the intramyocardial segment of the ramus interventricularis anterior and in segments proximal and distal to the bridge. We found the index was lowest in the myocardial bridge segment and was higher in both the proximal and distal segments, but highest in the proximal segments.
Some other workers [1, 9, 13] have made observations similar to ours. They also found that atherosclerosis was absent from the intramyocardial segment of the ramus interventricularis anterior but was intense proximally to the myocardial bridge. Masuda et al. [13] found that the atherosclerosis index of the segment 30-40 mm distal to the myocardial bridge did not differ much from that in the intramyocardial segment. Ishikawa et al. [32] have reported that the most intense atherosclerotic changes developed 2 cm proximal to the entrance of the intramyocardial segment. However, other workers [19-21] have made observations and drawn conclusions different from ours. They have concluded that atherosclerosis develops equally in the intramyocardial segment wall and in other parts of the descending artery and that the myocardial bridge has no influence on the development or localization of atherosclerotic changes. For example, Tomanovi?-Kokotovi? et al. [19] found atherosclerotic changes, predominantly of the type IV and V, in about 90% of intramyocardial segment walls.
Vanildo et al. [20], in autopsy examination of 30 human hearts, found atherosclerosis along the entire length of the anterior interventricular branch, while Winter et al.[21], using intravascular ultrasound, found atherosclerotic changes of lower intensity in the intramyocardial segment.
Possible mechanisms by which the myocardial bridge could lead to the development of atherosclerosis in the ramus interventricularis anterior have been put farther. Some scientists [33] have suggested that contraction of the myocardial bridge fibers disturbs coronary arterial hemodynamics. Others [34] have asserted that high blood pressure in the proximal segment, probably due to contractions of myocardial bridge fibers with reduction of the lumen diameter, causes retrograde blood flow and turbulence proximally. Turbulent blood flow, decreased shearing stress, and increased pressure in the proximal segment might injure the intima and contribute to the accelerated development of atherosclerosis [35].
Although myocardial bridges are present at birth, some of the bridges apparently cause no symptoms throughout a person’s lifetime, but in other persons the bridges may lead to symptoms of stable or unstable angina pectoris [22], myocardial infarction [23,24,25], pulse disorders [26,27], or sudden cardiac death [28,29]. Recent invasive diagnostic methods (quantitative coronary angiography and intravascular ultrasound) have shown that myocardial bridges cause not only reduction in systolic diameter of the arterial lumen, but also an average reduction of about 30% during the first third of diastole [17,30,31]. This diastolic reduction in luminal diameter perhaps might result in myocardial ischemia. On the other hand, contrary views exist about the influence of the bridges on descending artery wall structure and development of atherosclerotic change: Geiringer [8], from autopsy findings of 100 human hearts, emphasized that the intramyocardial segment intima was spared from atherosclerosis, an opinion shared by Ishii et al. [10]. Their histopathological study, with application of electron microscopy, showed that the intima of the artery under the myocardial bridge had only contractile smooth muscle cells and much spiral, twisted collagen in the interstitium. Endothelial cells in the intramyocardial segment were spindle-like and bulged along the axes of the blood vessel, i.e. in the direction of blood flow, which indicated that high shearing stress was present in the intramyocardial segment that acted to protect against development of atherosclerosis. Unlike endothelial cells of the intramyocardial segment, similar cells of the proximal segment had polygonal and flat shapes. The cells are distributed so that they resemble a low sidewalk, with spots affected by the low pulse pressure, which predisposes to atherosclerosis by enabling an intense transfer of lipids from blood to the subendothelial area of the blood vessel wall.
We believe that future studies should pay special attention to the influence of morphological properties of the myocardial bridge on the propensity for proximal atherosclerosis.
CONCLUSION
The presence of a myocardial bridge in the ramus interventricularis anterior is a risk factor for the development of atherosclerotic changes proximal to the entrance of its intramyocardial segment.
The changes are most prominent in the proximal segment of the vessel, 1.5-2 cm from its entrance into the myocardium.
This study conforms with the Declaration of Helsinki.

References:
|
1.
|
Ha?žiselimovi? H, Še?erov D, Rizvanbegovi? S. On myocardial bridges and loops in blood vessels of human heart. Fol Med Fac Med Univ Saraeviensis. 1974; 9: 19-38.
|
|
2.
|
Waller BF, Opr C, Slack JD et al. Anatomy, Histology and Pathology of Coronary Arteries: A Review Relevant to New Interventional and Imaging Techniques-Part IV. Clin. Cardiol. 1992; 675-687.
|
|
3.
|
Alegria JR, Herrmann J, Holmes DR, Lerman A, Rihal CS. Myocardial bridging. European Heart Journal. 2005; 26(12):1159-1168.
|
|
4.
|
Linzbach J. Die Bedeutung der Gefasswandfaktoren fur die Entstehung der Arteriosklerose. Verth Dtsch Ges Pathol. 1957; 41: 24-41.
|
|
5.
|
Osaka T, Ishii T, Hosoda Y. et al. The significance of myocardial bridge upon atherosclerosis in the left anterior descending coronary artery . J Pathol. 1986; 148: 279-291.
|
|
6.
|
Kosinski A., Grzybiak M. Myocardial bridges in the human heart: morphological aspects. Folia Morphol (Warsz). 2001; 60: 65–68.
|
|
7.
|
Luis Ernesto Ballesteros Acuna, Luis Miguel Ramirez Aristeguieta, Saldarriaga Bladimir Tellez. Morphological description and clinical implications of myocardial bridges:an snatomical study in colombians. Arq Bras Cardiol. 2009; 92(4): 242-248.
|
|
8.
|
Geirenger E. The mural coronary. Am Heart J. 1951; 41:359–368.
|
|
9.
|
Lee SS, Wu TL. The role of the mural coronary artery in prevention of coronary atherosclerosis. Arch. Pathol. 1972; 93(1): 32-35.
|
|
10.
|
Ishii T, Asuwa N, Masuda S, Ishikawa Y, Kiguchi H, Shimada K. Atherosclerosis suppression in the left anterior descending coronary artery by the presence of a myocardial bridge: an ultrastructural study. Mod Pathol. 1991; 4: 424–431.
|
|
11.
|
Ishikawa Y, Ishii T, Asuwa N, Masuda S. Absence of atherosclerosis evolution in the coronary arterial segment covered by myocardial tissue in cholesterol-fed rabbits. Virchows Arch. 1997; 430: 163-171.
|
|
12.
|
Ishikawa Y, Akasaka Y, Ito K et al. Significance of anatomical properties of myocardial bridge on atherosclerosis evolution in the left anterior descending coronary artery. Atherosclerosis. 2006; 186: 380-389.
|
|
13.
|
Masuda T, Ishikawa Y, Akasaka Y. et al. The effect of myocardial bridging of the coronary artery on vasoactive agents and atherosclerosis localization. J Pathol. 2001; 193: 408–414.
|
|
14.
|
Kawawa Y, Ishikawa Y, Gomi Y et al. Detection of myocardial bridge and evaluation of its anatomical properies by coronary multislice spiral computed tomography. Eur J Radiol. 2007; 61(1): 130-138.
|
|
15.
|
Zeina AR, Odeh M, Blinger J et al. Myocardial Bridge: Evaluation on MDCT, AJR. 2007; 188: 1069-1073.
|
|
16.
|
La Grutta, Runza G, Lo Re G et al. Prevalence of myocardial bridging and correlation with coronary atherosclerosis studied with 64-slice CT coronary angiography. Radiol Med. 2009; 114(7): 1024-1036.
|
|
17.
|
Ge J, Erbel R, Rupprecht HJ et al. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging. Circulation. 1994; 89: 1725–1732.
|
|
18.
|
Ge J, Jeremias A, Rupp A et al. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and Doppler. Eur Heart J. 1999; 20: 1707–1716.
|
|
19.
|
Tomanovi?-Kokovi? J, Teofilovski-Parapid G, Oklobdžija M et al. Uticaj pojave miokardnih premoš?enja koronarnih arterija na promjene strukture miokarda i zida koronarnih arterija. Vojnosanit Pregl. 2006; 63(2): 148-152.
|
|
20.
|
Vanildo ML, Jennecy SC, Tetso T. Myocardial Bridges and their Relationship to the Anterior Interventricular Branch of the Left Coronary Artery. Arq. Bras. Cardiol. 2002; 79(3): 84-93.
|
|
21.
|
Winter RJ, Kok WM, Piek JJ. Coronary atherosklerosis within a myocardial bridge, not a benign condition. Heart. 1998; 80: 91-93.
|
|
22.
|
Kulan K, Kulan C, Tuncer C, Komsuoglu B, Telatar M. Myocardial perfusion scintigraphy in a myocardial bridging of coronary artery. Clin Nucl Med. 1996; 21: 888–889.
|
|
23.
|
Vasan RS, Bahl VK, Rajani M. Myocardial infarction associated with a myocardial bridge. Int J Cardiol. 1989; 25(2): 240-241.
|
|
24.
|
Feldman AM, Baughman KL. Myocardial infraction associated with a myocardial bridge, Am Heart J. 1996; 111: 784-788.
|
|
25.
|
Zeina AR, Shefer A, Sharif D et al. Acute myocardial infraction in a young woman with normal coronary arteries and myocardiall bridging. British Journal of radiology. 2008; 81: 141-144.
|
|
26.
|
Farugui AM, Maloy WC, Felner JM et al. Symptomatic myocardial bridging of the coronary artery.Am J Cardiol. 1978; 41: 1305-1310.
|
|
27.
|
Dulk K, Brugada P, Braat S et al. Myocardial bridging as a cause of paroxysmal atrioventricular block. J Am Coll Cardiol. 1983; 1:965-969.
|
|
28.
|
Bestetti RB, Costa RS, Oliveira JM. Can isolated myocardial bridging of the left anterior descending coronary artery be associated with sudden death during exercise? Acta Cardiologica. 1991; XLVI: 27-30.
|
|
29.
|
Desseigne P, Tabib A, Loire R. Pont myocardique sur l’interventriculaire antérieure et mort subite. A propos de 19 cas autopsies. Arch Mal Coeur. 1991; 84: 511–516.
|
|
30.
|
Schwarz ER, Klues HG, vom Dahl J et al. Functional, angiographic and intracoronary Doppler flow characteristics in symptomatic patients with myocardial bridging: effect of short-term intravenous beta-blocker medication. J Am Coll Cardiol. 1996; 27: 1637–1645.
|
|
31.
|
Lovell MJ, Knihht CJ. Invasive assessment of myocardial bridges. Heart, 2003; 89(7): 699-700.
|
|
32.
|
Ishikawa Y, Akasaka Y, Fujiwara M et al. Anatomic properies of myocardial bridge predisposing to myocardial infraction. Circulation. 2009; 120(5): 376-383.
|
|
33.
|
Ishikawa Y, Kawawa Y, Kohda E, Shimada K, Ishii T. Significance of the anatomical properties of a myocardial bridge in coronary heart disease. Circ J. 2011;75(7):1559-66.
|
|
34.
35.
|
Ge J, Erbel R, Gorge G, Haude M, Meyer J. High wall shear stress proximal to myocardial bridging and atherosclerosis: intracoronary ultrasound and pressure measurements. Br Heart J. 1995; 73: 462–465.
Aleksandri? S, Parapid B, Jankovi? R et al. Miokardni mostovi: Od slu?ajnog nalaza do miokardne ishemije. Srce i krvni sudovi 2013; 32(2):110-120.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License