IJCRR - 9(5), March, 2017
Pages: 33-37
Date of Publication: 20-Mar-2017
Print Article
Download XML Download PDF
Effect of Sprouting Time on Antioxidant Properties of Trigonella foenum-graecum (Fenugreek) Seeds Available in Delhi-NCR Region
Author: Sadhna Jain1, Punita Saxena2, Heena Lamba3
Category: Healthcare
Abstract:Aim: Fenugreek seeds are popular for their diverse therapeutic benefits. Processing intervention to improve nutritional properties of such agricultural seeds is an age old practice. In the current study, sprouted fenugreek seeds have been analyzed for their potential use as a home based approach for the management of oxidative stress related disorders.
Methodology: Germinated seeds were dried at 60\?C, powdered and stored at 4\?C. The samples extract (10 mg/mL) in distilled water were then analyzed for Total Phenolic Content (TPC) and antioxidant activity by DPPH and FRAP. The results obtained for seven days were compared for significant differences using one-way ANOVA.
Results: The process of sprouting showed significant changes in the antioxidant properties of fenugreek seeds with the maximum activity observed on 7th day of sprouting. An increase ranging from 40% to 45% in TPC and antioxidant activity was observed on the seventh day of germination. A correlation analysis among the TPC and antioxidant activity (DPPH and FRAP) was also performed that confirmed a high value of R2 (0.965, 0.956) between the two.
Conclusions: Fenugreek seeds at seventh day of sprouting have the prospect to be used as nutraceutical for the management of oxidative stress related disorders.
Keywords: Total phenolic content, DPPH, FRAP, Antioxidant activity, Fenugreek seeds
Full Text:
INTRODUCTION
For many years mankind is using plant sources to alleviate or cure chronic illness like diabetes, cardio-vascular diseases, cancer, hypertension etc. Herbal plants are inherently rich in pharmacologically active compounds and hence herbal remedies are popular and used globally for their health benefits. Fenugreek or Trigonella foenum graecumis a legume and belongs to family ‘Fabaceae’ and is commonly called as “methi”. It is a herb cultivated for its seeds majorly in Mediterranean countries. In India, it is mainly cultivated in regions of Rajasthan (maximum production), Tamil Nadu, Gujarat, Madhya Pradesh, Punjab and Uttar Pradesh. Apart from the flavouring properties of its seeds, it has been able to mark its presence in ayurvedic system of medicines since ages, owing to its nutritional and therapeutic benefits. It is a rich source of fibres, proteins, vitamin A and C, iron and calcium. It is a highly recommended medicinal plant for treatments of various dysfunctions and diseases, as recorded in history of Ayurveda. Being rich in phytochemicals like phenols, flavonoids, alkaloids and tannins, it has been taggedas antidiabetic, anticarcinogenic, hypocholesterolemic, antioxidant, and immunological booster.
There is increased evidence of participation of free radicals in pathogenesis of various diseases like cancer, diabetes, ageing etc. Free radical is an atom of molecule with unpaired electron. Antioxidants on the other hand, are agents that scavenge free radicals and prevent or impedes cell oxidation by free radicals. Antioxidants can greatly reduce oxidative damage by neutralizing free radicals and thereby preventing damage to lipids, protein, enzymes, carbohydrates and DNA. Many studies have reported antioxidant properties of fenugreek seeds [1]. Fenugreek seed powder supplementation in diet has also been reported to reduce oxidative damage biomarkers in alloxan-diabetic rats [2]. Further polyphenols present in the seeds are known to prevent oxidative haemolysis and lipid peroxidation induced by hydrogen peroxide in vitro, in human erythrocytes[2].
Germination or sprouting is a processing intervention by which nutritional content of the crop can be enhanced significantly[3]. Sprouting has shown to improve the nutritional profile of fenugreek seeds and decrease the fibre content such that it gets digested and absorbed in the system more easily. Also, germinated fenugreek seeds have higher antioxidant content and enhanced antidiabetic effect than its boiled counterpart [4]. This effect was attributed to the release or higher bioavailability of bound antioxidants upon germination. Physical conditions such as light and temperature, genotype [5] and chemical composition of seeds have also been reported to be a cause of variation on antioxidant properties and germination of fenugreek seeds [6]. Few reports are available on the effect of germination on total phenol content and antioxidant properties of fenugreek seeds. These studies either employed use of natural elicitors like fish protein hydrolysates, lactoferrin and oregano extract for sprouting fenugreek seeds [7] or different fractions of the germinated seeds were evaluated for the phytochemical analysis [8]. However, very few studies have been conducted on antioxidant properties of whole aqueous extract of germinated fenugreek seeds of varieties available locally in Delhi/NCR region of India. Thus, present study is aimed to investigate the effect of germination on total phenolic content and antioxidant properties of fenugreek seeds in Delhi/NCR region for the purpose of using it as a nutraceutical, showing maximum potential of antioxidant activity under given conditions.
MATERIALS AND EQUIPMENTS
The materials used in the conduct of in vitro assays included
- Fenugreek seeds obtained from local market of Delhi/NCR region of India.
- Chemicals used included Folin-Ciocalteu reagent, 2, 2-diphenyl-1-picrylhydrazyl (DPPH), sodium carbonate and aluminum chloride, Ascorbic acid, Gallic acid, Potassium ferricyanide, trichloroacetic acid, Ferric chloride.
- Equipments used for biochemical analysis were Electric air draught oven (Universal Oven sourced from Narang Scientific Works), spectrophotometer (Systronics Visican 167)
GERMINATION AND PREPARATION OF EXTRACTS
Soaking and germination
Fenugreek seeds in eight portions of 50 g each were soaked in 70% ethanol solution for 15 minutes at room temperature for disinfection. Soaked seeds were then washed with tap water and distilled water. Washed seeds were then soaked in distilled water (1:10 w/v) for 12 h at room temperature. The presoaked seeds were again washed in distilled water and kept for germination on flat trays lined with moist paper towel. The trays were covered with aluminum foil for dark germination. The germinating seeds were kept moist with distilled water and germinated for 7 days. Sprouted seeds after each day starting from first day were frozen to stop further germination. After thawing at room temperature, seeds were dried in an electric air draught oven at 60°C for 48 hrs. Dried and sprouted seeds were ground in an electric grinder, sieved and stored in plastic bottle container at 4°C for further analysis.
Stock solution of extracts
Extracts of germinated seeds of each day were prepared by dissolving powder at a concentration of (10 mg/mL) in distilled water and filtered through layers of filter paper and assays were carried out with freshly prepared solutions.
BIOCHEMICAL AND STATISTICAL ANALYSIS
Total phenolic content (TPC)
Antioxidant activity of different extracts was determined by estimating TPC following the method adopted by Musa et al. [9]. Approximately 0.4 mL distilled water and 0.5 mL diluted Folin-Ciocalteu reagent was added to 100 µL fenugreek seeds extracts. The samples were set aside for 5 min and 1 mL 7.5% sodium carbonate (w/v) was added. The absorbance of sample was then taken at 765 nm using a spectrophotometer after 2 hrs. The calibration curve of Gallic acid was used for the estimation of sample activity. The result was recorded in terms of mg of Gallic acid equivalents per 100g of dry powder (mg GAE/100 g of dry powder).
DPPH radical scavenging activity
The antioxidant activity was assessed using a 2, 2-diphenyl-1-picrylhydrazyl (DPPH) scavenging system [9]. The stock solution was obtained by dissolving 40 mg DPPH in 100 mL methanol and then stored at -20°C till further use. Approximately 350 mL stock solution was mixed with 350 mL methanol to obtain the absorbance of 0.70 ± 0.01 unit at 516 nm using a spectrophotometer. In the dark, 100 µL germinated fenugreek seeds extracts was mixed with 1 mL prepared methanolic DPPH solution. The samples were stored overnight and scavenging activity was determined based on the following equation:
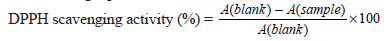
Where A denotes the measure of absorbance.
Antioxidant activity (FRAP)
Antioxidant assay was estimated by ferric reducing antioxidant power method [10]. To 2.5 mL of extract, 1mL of 0.2 M phosphate buffer (pH 6.6) and 1mL of 1 % potassium ferricyanide was added. The reaction mixture was incubated in water bath at 50°C for 20 minutes. Reaction mixture was then rapidly cooled. In order to stop the reaction, 2.5 mL of 10% trichloroacetic acid was added. 2.5 ml of the sample was taken and 2.5 mL of distilled water and 0.5 mL of 0.1 % ferric chloride solution was added. The colour of the mixture changed to green. The mixture was allowed to stand for 10 minutes. Absorbance was recorded at 593 nm using spectrophotometer. The blank was performed using reagent blank and Ascorbic acid was taken as standard. The result was recorded in terms of mg of Ascorbic acid equivalents per 100g of dry powder (mg AAE/100 g of dry powder).
Statistical analysis
The results obtained for seven days were tested for significant differences using one-way ANOVA on Microsoft Excel.
RESULTS AND DISCUSSIONS
Data obtained by experimental results were expressed as the means of three independent experiments. The results obtained for seven days were compared for significant differences using one-way ANOVA on Microsoft Excel. Total phenolic (TP) content, Antioxidant activity by DPPH and FRAP are presented in Table 1.
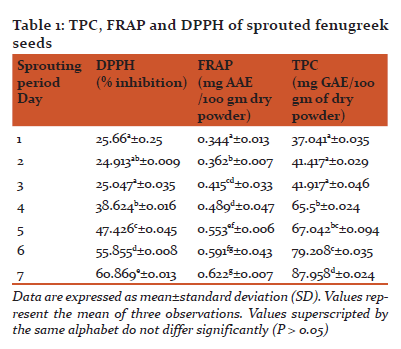
The process of sprouting showed significant changes (P≤0.05) in the antioxidant properties of fenugreek seeds with the maximal activity observed on day 7 of sprouting.
Total Phenol Content (TPC)
Figure 1 shows the variation in the total phenolic content of sprouted fenugreek seeds grown over a span of seven days. TP content was seen to be increasing with the days from 37.041 mg/100 gm of dry powder on the 1st day to 87.958 mg/100 gm of dry powder on the 7thday of germination. Total phenolic content increased significantly (P≤0.05) from the 1st day till 7th day. The maximum content was observed on the seventh day (87.958±0.024 mg/100 gm DW). After the seventh day, a slight fungal growth was observed in the sample and hence no evaluations were made beyond seventh day of germination. Interestingly, seeds used in the sample showed absence of flavonoids in spite of high TP content and antioxidant activities. This could be due to an increase in other phenolic compounds present in fenugreek seeds. As can be seen from the graph, there is no significant difference (P>0.05) in the TP content on day 5 and 6 but there is a significant difference in the TP content on day 6 and 7(P≤0.05).
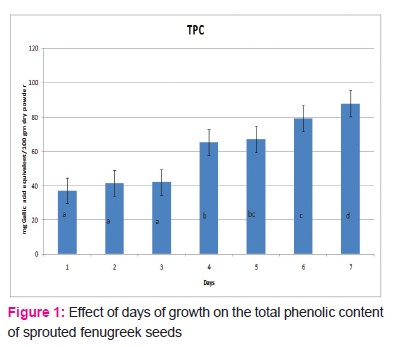
Ferric Reducing Antioxidant Power (FRAP)
Figure 2 shows the ferric reducing antioxidant power values for sprouted fenugreek seeds over seven days of germination. The values showed a significant increase (P≤0.05) from 0.344±0.013 mg/100 gm of dry powder on the 1stday to 0.622±0.007 mg/100 gm of dry powder on 7th day. The maximum activity for FRAP was observed on 7th day of germination. However, the values on day 6 and 7 are not statistically significantly different (P>0.05). This means the optimal ferric reducing antioxidant power does not change whether the seeds are used after 6 or 7 days of germination.
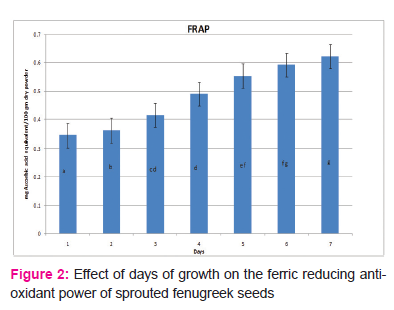
DPPH
Figure 3 shows the free radical scavenging activity values determined by DPPH over 7 days of germination of fenugreek seeds. This activity was measured in terms of % inhibition exhibited by the sample observations against controlled observations. These values ranged from 25.66 % on the 1stday to 60.869% on 7th day of germination. Sprouted seeds showed a significantly (P≤0.05) higher radical scavenging activity over the seven day period of germination. This characteristic helps in managing oxidative stress related degenerative disorders and reduces the risk of age related degeneration. It was observed that there was no significant difference (P>0.05) in the DPPH values during the first three days of germination. However, the DPPH values were significantly different(P≤0.05) on 5th, 6thand 7th day of germination and the maximum was observed on 7th day. Sprouting of seeds is known to produce various metabolic activities in seeds and an increase in antioxidant activity is one such change. This change could be attributed to the amalgamation of various compounds.
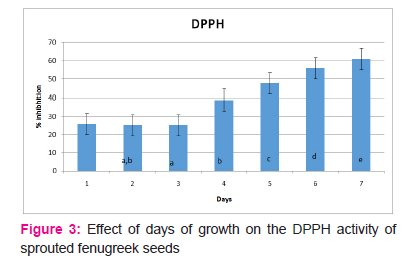
Hence, it can be concluded that day 7 is the optimal day of germination for fenugreek seeds to be used for nutraceutical purposes as they show maximum total phenol content and antioxidant activities on this day.
Correlation:
A correlation analysis among the TPC and antioxidant activity (DPPH, FRAP) was also performed. Table 2 below shows a high value of R2 showing a significant correlation between the two. Studies [9] have shown that antioxidant activity is related to the active component irrespective of the extraction techniques and solvents used. In the present study, it can be concluded that the process of germination also preserves this property of high correlation.

CONCLUSIONS
The results of the study showed that the process of sprouting increases the Total Phenolic Content, ferric reducing antioxidant power and free radicals scavenging activities in fenugreek seeds significantly (P≤0.05). The difference in the activities was highly significant between the third day and the sixth day of germination. Thus, for the best results the seeds should be consumed after at least six days of germination. Total phenolic content showed a good correlation with DPPH and FRAP. Thus, it can be concluded that sprouted fenugreek seeds have a high potential to be used as a cost effective approach to be used as nutraceutical for the management of oxidative stress related disorders in a developing country like India.
FUTURE PROSPECTS OF THE STUDY
The study and the experiments were conducted by in-vitro method only. No clinical testings were done. The study can be extended by involving dieticians and clinical experts to analyse the efficacy of the results obtained.
Conflict of Interest
The authors of this manuscript declare that they have no conflict of interest.
Acknowledgements
The authors are thankful to Dr. Ritu Verma, Head, Cell Biology Division, Dabur Research Foundation, India for her guidance in carrying out this study and University of Delhi, India for funding this project under the Innovation Projects Scheme 2015-16. Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors/editors/publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
References:
[1] Wani SA, Kumar P., Fenugreek: A review on its nutraceutical properties and utilization in various food products. J Saudi Soc Agr Sci 2016; http://dx.doi.org/10.1016/j.jssas.2016.01.007
[2] Ravikumar P, Anuradha CV. Effect of fenugreek seeds on blood lipid peroxidation and antioxidants in diabetic rats. Phytother Res 1999;13(3):197-201.
[3] Pandey H, Awasthi P. Effect of processing techniques on nutritional composition and antioxidant activity of fenugreek (Trigonella foenum-graecum) seed flour. J Food Sci Tech 2015;52(2):1054-1060.
[4] Naidu MM, Shyamala BN, Naik JP, Sulochanamma G, Srinivas P. Chemical composition and antioxidant activity of the husk and endosperm of fenugreek seeds. LWT-Food Sci Tech 2011;44:451-456.
[5] Singh KP, Nair B, Naidu AK. Contribution of fenugreek (Trigonella foenum graecum L.) seeds towards the nutritional characterization. J Med Plants Res 2013;7(41):3052-3058.
[6] Pour AP, Farahbakhsh H, Saffari M, Keramat B. Effects of Seed Priming on Germination and Seedling Growth under Salinity Stress in Fenugreek. Int JAgr Sci 2012;9(12):779-786.
[7] Randhir R, Lin YT, Shetty K. Phenolics, their antioxidant and antimicrobial activity in dark germinated fenugreek sprouts in response to peptide and phytochemical elicitors. Asia Pac J Clin Nutr 2004;13(3):295-307.
[8] Dixit P, Ghaskadbi S, Mohan H, Devasagayam TP. Antioxidant properties of germinated fenugreek seeds. Phytother Res 2005;19(11):977-983.
[9] Musa KH, Abdullah A, Jusoh K, Subramaniam V. Antioxidant activity of pink-flesh guava (Psidium guajava L.): effect of extraction techniques and solvents. Food Anal Method 2011;4(1):100-107.
[10] Patel A, Patel A, Patel NM. Estimation of flavonoid, polyphenolic content and in-vitro antioxidant capacity of leaves of Tephrosia purpurea Linn.(Leguminosae). Int J Pharm Sci Res 2010;1(1):66-77.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License