IJCRR - 9(5), March, 2017
Pages: 26-32
Date of Publication: 20-Mar-2017
Print Article
Download XML Download PDF
Assessment and Comparison of Liver Functions in Leprosy
Author: G. P. Dhavalshankh1, A. G. Dhavalshankh2, S. Gaurkar3
Category: Healthcare
Abstract:Leprosy is chronic infectious disease of man, caused by Mycobacterium leprae, affecting peripheral nerves, skin and sometimes other tissues. Hepatic involvement is seen in all stages of the leprosy, more so in lepra reactions.
Aim: The present study was undertaken to evaluate hepatic status by studying the various liver function tests in leprosy patients as well as in patients of lepra reaction.
Methodology: Sixty untreated leprosy patients (30 Multibacillary, 30 Paucibacillary) with duration of illness varying from one month to three years were considered. Hepatic functional status was evaluated by estimation and comparison of variations in the levels of liver enzymes, proteins (Albumin, Globulin) and Australia antigen in paucibacillary, multibacillary leprosy and type I&2 lepra reactions.
Results: Deceased levels of serum albumin were noted in all forms of leprosy except in type II reaction while serum globulins were decreased only in paucibacillary leprosy. Raised levels of SGOT were found in all forms of leprosy including lepra reactions. However SGPT was significantly raised in type II lepra reaction. Serum bilirubin was raised in type II lepra reaction while raised levels of serum alkaline phosphatase were observed in type I lepra reaction. Serum cholesterol levels were decreased in all forms of leprosy except in type I reactions.
Conclusion: We found that liver is significantly affected in leprosy and in lepra reactions. Assessment of liver functions is useful to measure the severity of affection of the liver in leprosy and for monitoring the patients on antileprosy treatment..
Keywords: Leprosy, Liver Function Tests (LFT), Multibacillary (MB), Paucibacillary (PB)
Full Text:
INTRODUCTION:
Leprosy is chronic infectious disease of man, caused by mycobacterium leprae, affecting peripheral nerves, skin and sometimes other tissues.1 In individuals having no cell mediated immunity against mycobacterium leprae, a widespread clinical form of leprosy is seen. This form is called lepromatous leprosy. The continuous bacillemia of lepromatous leprosy, estimated at 105 organisms/ml blood, ensures the constant bombardment of internal organs by mycobacterium leprae. The reticuloendothelial system acts as a filter to the circulating bacteria which accumulate in macrophages in the liver, spleen, bone marrow and several groups of lymph nodes especially in lepromatous leprosy.
The liver lesions in lepromatous leprosy are fairly common and are well described.2,3 Histopathological examination shows prominent Kupffer cells and numerous miliary lepromas.4 Involvement of liver, of a milder nature and degree is also seen in other types of leprosy such as tuberculoid leprosy. Tuberculoid granulomas in the liver of leprosy patients are known to occur especially during the reactive phase and have been well described.5 The involvement of liver in leprosy is well reflected in serum enzymes denoting liver function which have been found to be elevated mainly in lepromatous leprosy. There are reports of increased serum bilirubin, reversal of albumin globulin (A: G) ratio5 and increased SGOT6 and also SGPT7, increased serum gamma globulins6 but decreased serum cholesterol.8
The aim is to collect a comprehensive data regarding biochemical parameters of liver in leprosy with comparison of the collected data between the two groups of leprosy (i.e. Paucibacillary and multibacillary) and lepra reactions (Type I and Type II) with the control.
MATERIALS AND METHODS:
The present study was conducted over a period of two years in 60 patients attending outpatient department of Skin and Sexually Transmitted Disease (STD) in a government hospital, Kolhapur. After ethical consideration and written consent, patients without prior history of leprosy or prior history of leprosy treatment i.e. freshly diagnosed leprosy cases were chosen from the outpatient department depending on their willingness to undergo investigations.
A detailed history was taken to rule out chronic alcoholism, recent history of jaundice, liver disease or recent intake of any hepatotoxic drugs. Patients with such history were excluded from the study. Remaining patients were then subjected to careful clinical examination to determine the extent of the disease. The clinical type of the disease was determined according to Ridley and Jopling’s classification9 (1966) and findings were entered in proforma. After that, patients were subjected to special investigation called “skin clip”.
Skin clip
The skin clip was done by ‘Slit and Scrape’ method of Wade. Smears were made from suspected lesions as well as from sites commonly affected in lepromatous leprosy – forehead, ear lobules, chin, extensor aspect of forearm, buttocks, nasal cavity etc. The smear was stained by Ziehl-Neelsen method for staining for acid fast bacilli.
Recording of smear reports
About 50 to 100 fields were examined with oil immersion lens and results were noted as positive or negative. In cases of positive finding, results were recorded as follows:
6+ Very numerous – more than 1000 bacilli; or globi per oil immersion field.
5+ Numerous – 100 to 1000 bacilli per oil immersion field.
4+ Moderate – 10 to 100 bacilli per oil immersion field.
3+ Few – 1 to 20 bacilli per oil immersion field.
2+ Very few – 10 to 100 bacilli per entire slide (100 fields).
1+ Rare – 1 to 10 bacilli per entire slide (100 fields).
Bacteriological Index (BI) was calculated by adding the degree of positivity of all smears and dividing the total by number of smears examined. Those patients with positive bacteriological index were grouped under multibacillary group and those with negative bacteriological index in paucibacillary group. In each group, 30 patients were included to make a total of 60 patients for our study. Also 30 normal healthy individuals were chosen as control group. All these 90 patients were subjected to special investigation of liver function tests.
Liver function tests: 10
About 20 ml of blood was collected, usually on the day of admission by venepuncture using aseptic technique following liver function tests were carried out.
- Total plasma proteins, serum albumin and globulins (Biuret method for total proteins and albumin by bromocresolgreen method).
- Serum bilirubin (Malloy and Evalyx method).
- Serum glutamic pyruvic transaminase (SGPT) (Calorimetric method of Reitman and frankel).
- Serum glutamic oxaloacetic transaminase (SGOT) (Calorimetric method of Reitman and Frankel).
- Serum alkaline phosphatase (king Armstron method).
- Serum cholesterol (ferroham method).
- Australia antigen (Latex Agglutination).
Due to difficulty in performing electrophoresis of protein fractions to find out differential proteins (alpha, beta, gamma proteins) in our institute, this was not done.
Statistical method: Independent sample’t’ test was used to compared the data. P < 0.05 was considered as significant and evaluated by ANNOVA method.
DISCUSSION
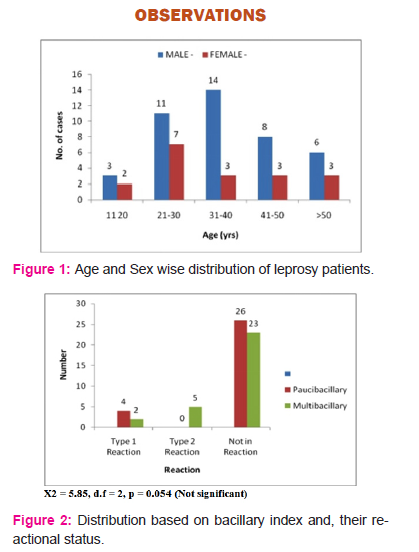
In this study sixty cases of freshly diagnosed patients of leprosy were included. The ratio of male to female patients was 2.33:1 which corresponds to normally found 2:1 ratio of male leprosy patients to female leprosy patients in our population. Out of 60 cases, 42 were male patients and 18 were female patients. Majority of the patients were belonging to age group of 20 to 40 years. The youngest patient was 16 years old while the oldest was 75 years. (Fig 1)
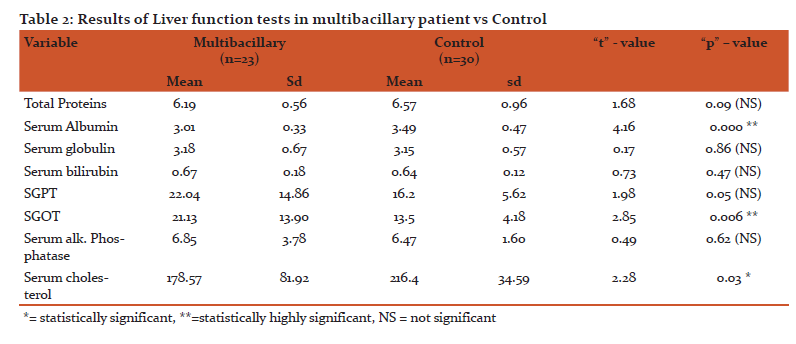
The two groups studied include 30 paucibacillary leprosy patients in first group and 30 multibacillary leprosy patients in the second group. In the 30 cases of paucibacillary leprosy 29 were borderline tuberculoid (BT) and 1 was of tuberculoid tuberculoid (TT) leprosy. In second group of 30 cases of multibacillary patients, 2 were borderline borderline (BB), 21 were borderline lepromatous (BL), 7were lepromatous lepromatous (LL) patients. This shows statistically highly significance. (Fig 2)
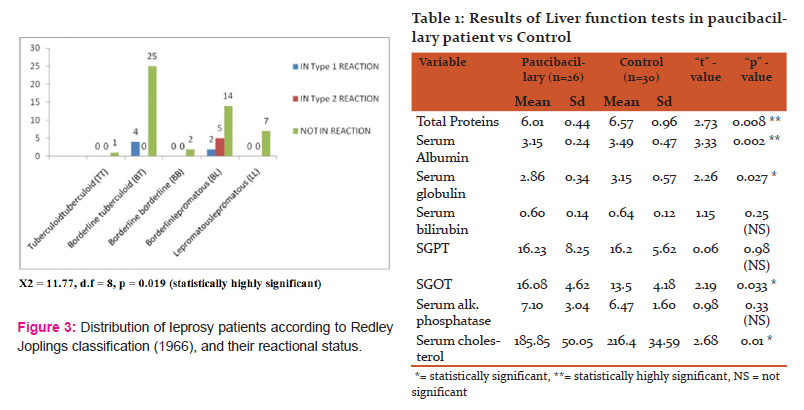
Out of these 60 patients, 11 patients were undergoing lepra reaction– 6 undergoing type I and 5 undergoing type II lepra reaction. In type I reaction, 4 were BT and 2 were BL leprosy cases. In type II (erythema nodosum leprosum) reaction, 5 cases of BL leprosy were studied. Here not any difference observed between type I and type II reaction in both groups of leprosy. (Fig 3)
LIVER FUNCTION TESTS IN LEPROSY
Plasma proteins (Total serum proteins, Albumin and Globulins):
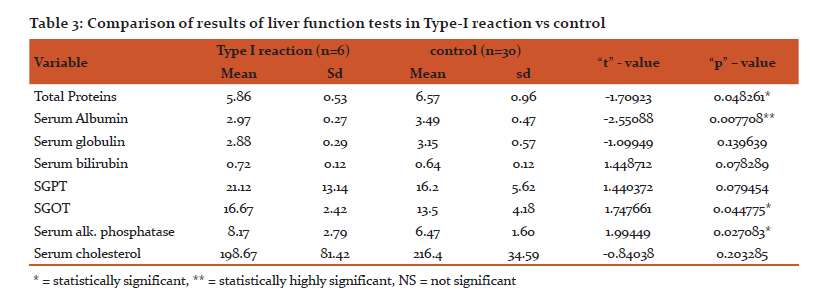
In this study, total serum proteins with standard deviation (sd) of 0.44 in paucibacillary leprosy patients as comparedd to 0.96 in control group giving statistically highly significant ‘p’ value. In multibacillary group serum total proteins with sd 0.56 which was very near to that in control group with sd 0.96 with statistically non significant p value. Thus we found that total serum proteins in normal range in multibacillary leprosy patients but significantly decreased in paucibacillary.
In our study serum albumin and globulin levels in paucibacillary leprosy patients were 3.154 gm% and 2.857 gm% respectively which were 3.49 gm% and 3.15gm% respectively in healthy control group. However, the mean serum albumin and globulin levels in multibacillary leprosy patients were 3.01 gm% and 3.18gm% respectively indicating that in paucibacillary serum albumin and globulin decrease is highly significant statistically. But in multibacillary leprosy there is highly significant decrease in albumin level but not significant with globulin. (Table 1 & 2)
Gupta et al11 recorded fall in total proteins in lepromatous type however we recorded significant fall in total proteins in paucibacillary leprosy but not in multibacillary type. M Swathi12 found the lowering of A/G ratio in leprosy cases which was statistically significant when comparedd to controls. But Shivde and Junnarkar et al7 and Nigam et al6 found normal or raised total proteins. Increase in total proteins, lowered serum albumin and raised globulin values were recorded by Kinnier and Davison13 and Gharpuray et al14. Gupta and Gupta15 states that decrease in total proteins and albumin are due to chronic destructive nature of the disease and liver involvement. It is well known that albumin synthesis takes place in hepatic cells whereas in globulin synthesis, both plasma cells and lymphocytes participate. Therefore one or more factor like decreased albumin synthesis due to hepatic dysfunction. However significant decrease in serum globulin is not detected due to stimulation of reticuloendothelial system leading to globulin synthesis.
In our study with type I reaction patients total serum proteins and albumin was significantly decreased but that of globulin is not statistically significant as comparedd to control. In type II reaction patients statistically significant changes were not observed in all three levels. In contrast to our observations, Patnaik J.K.et al16 observed altered albumin to globulin ratio in patients with lepra reaction. Similar findings observed by Ischikara’s17 in erythema nodosum leprosum (type II) are interesting. During the acute attack, globulins (particularly gamma) levels were very high and they came down after acute antibody phenomenon. Type I reaction is because of change in cell mediated immunological status hence there is hardly any change in humoral immunity so immunoglobulin production is not increased. In erythema nodosum leprosum, there is rapid destruction of lepra bacilli and antigenic load is more in circulation. This leads to antibody production and formation of immune complexes: this explains slight increase in globulin level in type II reaction as compared to type I reaction in our study.
Serum Enzyme Estimations in Leprosy (SGPT, SGOT):
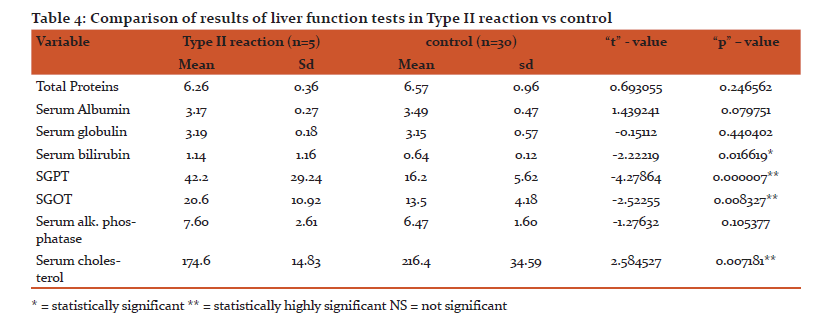
In the present study SGOT was significantly raised in paucibacillary as well as in multibacillary leprosy patients but there were not statistically significant alterations in SGPT levels. (Table 1&2) There was highly significant rise in the levels of SGPT in type II reaction patients as compared to type I reaction patients. In all the forms of leprosy including lepra reactions significant increased levels of SGOT were noted. (Table 3 &4)
Teresa et.al18 reported that there is raised SGOT and SGPT in lepromatous leprosy which correlates with our study. Nigam et al6 found increase in values of SGOT and SGPT in all types of leprosy. Gharpuray et al14 found increase in SGPT levels in 7 out of 20 tuberculoid cases, 7 out of 10 lepromatous cases and normal value in 8 dimorphous cases. Mohanty et al19 found increase in levels of both SGPT and SGOT in 14 out of 24 lepromatous patients and 5 out of 8 patients with lepra reaction.
Balkrishnan20 found more increase in patients with lepra reaction than lepromatous patients. Levels of SGPT were 34 and 30 IU/L and of SGOT were 52 and 32 IU/L respectively which correspond to our study. Kinnier and Davison13 and Shivde and Junnarkar7 observed a rise in serum transaminase activity in leprosy patients especially in lepromatous group which corresponds to our study.
The transaminases, serum glutamic pyruvic transaminase (SGPT) and serum glutamic oxaloacetic transaminase (SGOT) are present in substantial amount in liver and other sites like skeletal and cardiac muscle, pancreas and kidney. Normally they are just detectable in plasma. SGPT estimation is more sensitive than SGOT estimation. Estimation of these enzymes in leprosy is of particular importance since involvement of liver and skeletal muscle is frequently encountered in good number of leprosy cases mainly of lepromatous leprosy.6, 19
In different studies it is stated that slightly increase in these enzyme levels is due to skeletal muscle involvement in lepromatous leprosy. Nigam et al6 quoted this increase may be due to hepatic dysfunction and muscular involvement. Mohanty et al’s19 impression was that increase indicates subclinical involvement. Balkrishnan20 concluded that the increase in values in reaction may be due to breakdown of liver tissue in reaction. Shivde and Junnarkar7 quoted that rise in SGPT in lepromatous leprosy indicated a toxic effect of leprosy bacilli to hepatic cells. The values were especially high in those cases with portal cirrhosis and in those harboring miliary lepromas in the liver.
Alkaline Phosphatase:
In our study, significant rise in serum alkaline phosphatase was seen only in type I reaction patients, however normal levels were recorded in both the forms of leprosy and in patients with type II reaction.
Kappor21 and Mukharjee22 found increased levels in lepra reactions than lepromatous cases which correlates with our findings. Balkrishnan20 and Nigam et al6 found increased values more commonly in lepromatous type. According to Dhopale and magar23 values of serum alkaline phosphatase are normal in early cases and increase with severity of disease. They found more values in tuberculoid type than lepromatous type which correlates with our study. Ischihara17 also found increased value in all types of leprosy. This increase in levels of serum alkaline phosphatase may indicate subclinical hepatic involvement and it higher values in reaction may be due to destruction of liver tissue during reaction.
Serum Bilirubin
In the present study, serum bilirubin levels were significantly raised in type II reaction but no significant rise was noted in paucibacillary, multibacillary and in type I reaction patients.
Dhopale A. M. and Balkrishnan S.24 observed increased values of serum bilirubin levels in advanced stages of lepromatous leprosy. Nigam et al6 also observed hyperbilirubinaemia chiefly in lepromatous leprosy. Dhopale A.M. and Balkrishnan24 stated that the term bilirubin is generally employed to designate the several forms of iron free pigment present in the blood which are ultimately derived from the breakdown of hemoglobin. It is important to bear in the mind that bilirubin level in the blood is probably maintained at normal by different excretory mechanisms. The blood is cleared of bilirubin so long as sufficient normal functioning of hepatic tissue remains.
Serum Cholesterol
In our study of paucibacillary patients as well as multibacillary patients shows significant decrease in serum cholesterol value and highly significant decrease in type II reaction but not significant in type I reaction .
Our findings correspond to the findings of following studies. Robins et al8 and Gupta et.al25 (2002). They found low serum cholesterol values in patients of leprosy as compared to the normal controls, whereas Dhopale A.M. and Balkrishnan24 observed lowering of serum cholesterol levels in lepromatous leprosy. Dhopale and Magar23 observed that reduction of serum cholesterol levels was proportional to the severity of the disease. However K.C. Nayak et al26 in their study observed normal values for serum cholesterol in all groups of leprosy patients.
Robins et al8 found no cause for low serum cholesterol values in leprosy patient. However Dhopale A. M. and S. Balkrishnan24 stated that changes in serum cholesterol in leprosy patients are more in keeping with the usual cholesterol metabolism and excretion; and suggests that low serum cholesterol levels in lepromatous leprosy is due to the hepatic involvement. CM Nwosu and SNN Nwosu27 observed higher cholesterol level in lepromatous leprosy and also mentioned that patient have serum cholesterol levels in abnormal range this may predisposed to enhanced atherogenesis and increased cardiovascular morbidity.
Australia Antigen (Au Ag)
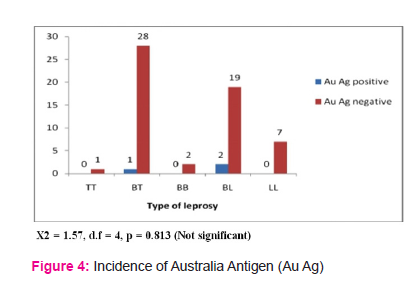
In the present study only one patient from borderline tuberculoid and 2 patients from borderline lepromatous leprosy were positive of Australia antigen. Thus incidence of Australia antigen in the present study is 5%.our study data correlate with observations of SK Sinha et.al28. Tin et al 29 in their study observed positivity for Austalia Antigen only in 2 patients in study group of 75. Blumberg et al30 in 1967 found that there is increased positivity for AuAg and in 1970 they found 6.2 % incidence in lepromatous and 2.5 % in tuberculoid leprosy in South India. Datta R. N. et al31 demonstrated high incidence of HbsAg in 8.1 % with no clinical manifestation of hepatitis but it was not found in tuberculoid leprosy cases. Nuti et al35 had found very high incidence of 24.4% in lepromatous leprosy (175 cases) and 11.5 % in tuberculoid leprosy (87 cases). Chakravarti et al32 had given incidence of HbsAg in lepromatous as 3.8% (234 cases) and in tuberculoid as 2.5% (431 cases). Kelkar et al33 found increased incidence in tuberculoid (6.3%) than lepromatous (5%) leprosy. Various other authors have given incidence upto 4%, slightly more in lepromatous than in tuberculoid leprosy.
. Datta R. N. et al31 described this positivity of HbsAg to poverty and hot climate. He also suggested that the increased incidences of positive AuAg in lepromatous leprosy as compared with tuberculoid leprosy may be due to decreased cell mediated immunity in patients of lepromatous leprosy. According to Kelkar et al33 this association of Australia antigen was merely reflection of opportunity for infection and stay in hospital. Because of decrease in cell mediated immunity in patients with lepromatous leprosy, they are unable to get rid of hepatitis – B virus once it gets entry in the body.
K. C. Nayak et al26 in study of 50 patients of various subtypes of leprosy and 25 healthy control, for detection of Australia antigen concluded that incidence of Australia antigen in both groups were zero. No relationship was established between hepatic lesion, Australia antigen and liver function test. They could not find any relationship between leprosy and HbsAg. These authors proposed genetic hypothesis that patients who are homozygous for a gene designated ‘AU’ are more susceptible to chronic infections with AU (I) virus, than individuals with alternate phenotypes as a consequent detectable Australia antigen in their blood. In their study patient represented as identical socio-economical group of population. The chance of infective organism in a community living in a residential home is always more which might be the reason for the higher incidence of HbsAg in lepromatous cases in Blumberg’s study.30
Genetic hypothesis states that presence of Australia antigen is determined by gene which is autosomal recessive. The individual homozygous for the gene may have detectable Australia antigen. Bearers of this gene may be more susceptible to certain illness like leukaemia, viral hepatitis, lepromatous leprosy etc. If this hypothesis is correct, then the population having both the gene and mycobacterium leprae are more likely to have impairment of their immunological mechanisms and so are more likely to have lepromatous leprosy than tuberculoid leprosy.
CONCLUSION:
In concluding, we found that liver is significantly affected in both the types of leprosy (Paucibacillary and Multibacillary) and in lepra reactions (Type I and Type II). AS the drugs used in the treatment of leprosy are known to be hepatotoxic, assessment of liver functions is useful to measure the severity of affection of the liver in leprosy and for monitoring the patients on antileprosy treatment.
ACKNOWLEDGEMENT:
Authors acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript. The authors are also grateful to authors / editors / publishers of all those articles, journals and books from where the literature for this article has been reviewed and discussed.
SOURCES OF FUNDING: None
CONFLICT OF INTREST: None
References:
- Bryceson ADM. Leprosy, 3rd edition, English Language Book Society, Churchill Levingstone, pg 1, 1990.
- Fite GL. Leprosy from histopathological point of view. Arch Path. 35 : 475-481, 1943.
- Mitsuda K,Ogawa M. A study of 150 autopsis on cases of leprosy. Lnt J Lepr.5:53-60,1937.
- Hastings RC. Leprosy, 2nd edition, Churchill Livingstone,Edinburgh London Madrid Melbourne New York and Tokyo, 1994.
- Karat ABA, Job CK and Rao PSS. Liver in leprosy, histopathology and biochemical findings. BMJ 307-310, 1971
- Nigam P, Ayala SG,Goyal BM,Joshi LD, Samuel KC. Leprous hepatitis, clinical and pathological study. Lepr in lndia 50:185, 1978.
- Shivde AV, Junnarkar RV. Seurm transaminases activity in leprosy in relation to liver damage. Int J Lepr. 35: 366-375, 1967.
- Robins K, Vijaykumar T, Gopinath T, Vasudevan DM. Liver in leprosy functional changes. Lepr in India. 52: 416, 1980.
- Ridley DS. Classification of leprosy-A window on leprosy, edited by BR Chatterjee, Pg 112, 1978.
- Wooten IDP. Microanalysis in medical biochemistry. 4th edition, J and A Churchill Ltd, London.
- Gupta SC, Sinha SN, Sharma D ,Bajaj AK, Bisht D, Malhotra TN. Serum proteis and Immunogiobulins in Leprosy. Int J Lepr. 46:9-14, 1978.
- M swathi . A Study of Liver Function Tests in leprosy, Indian journal of leprosy 86(4):155-9 · September 2015.
- Kinnier AA and Davison AR. Hormone excretion and liver function in gynecomastia of Leprosy. Int J Lepr. 25 : 110-118,1957.
- Gharpuray SM, Gharpuray MB, Kelkar SS. Liver functions in leprosy. Lepr in India . 49 : 216, 1977.
- Gupta RM,Gupta SC, Singh G,Khanna S. Immunoglobulins in leprosy. Int J Lepr. 46 : 342,1978.
- Patnaik JK, Saha PK, Satpathy SK, Das BS, Bose TK. Hepatic morphology in reactional status of leprosy. Int J Lepr. Other Mycobact Dis. 57 (2) :499-505, 1989.
- Ischikara S. A study of serum proteins in leprosy. Int J Lepr. 21 :187-189,1955.
- Teresa c. A. Ferrari, marcelo g. Arauâ jo & maria m. F. Ribeiro. Case report Hepatic involvement in lepromatous leprosy. Lepr Rev. 73, 72-75, 2002.
- Mohanty HC and Murhy RS. Serum transaminases in leprosy. Lepr in lndia 45:163 ,1973
- Balkrishnan S. Biochemical aspects of reactional states in leprosy. Lepr in India. 48 : 406, 1976
- Kappor KK, Gupta SH. Serum cholesterol and alkaline phosphatase in different types of leprosy. Lepr in India. 46; 152, 1974.
- Mukharjee A. Ghosh S. Liver function and coagulation factors in leprosy. Lepr in India. 45:19, 1973.
- Dhopale AM, Magar NG. Studies in blood chemistry of leprosy. Lepr in India. 34:299, 1962.
- Dhopale AM and Balkrishnan S. Liver function tests in leprosy. Ind J Med Res. 56 : 1552-1558, 10th Oct 1968.
- Gupta Anju , Ravindra V Koranne, Nancy Kaul. Study of serum lipids in leprosy. Indian Journal Of Dermatology, Venerology And Leprosy . : 68 (5) ;262-266: 2002
- Nayak KC Gupta RK,Agawal TD,Chadda VS and Krishnakumar K. A study of incidence of Australia antigen and derangement in Live Function tests in leprosy. lnd J Lepr .61:23-30,1989.
- CM Nwosu, SNN Nwosu. Abnormalities in Serum Lipids and Liver Fuction in Nigeria Patients with Leprosy. Journal of Medical Investigation and Practice.Vol.2: 5-10:2001
- SK Sinha, PP Banerjee, ML Bangal, GC Saha. Australia-antigen in lepromatous leprosy patients. Indian journal of dermatology. 31 (1) :36-37 ;1986
- Tin shwe and a. J. Zuckerman. Australia antigen and antibody in British patients with leprosy. J. clin. Path., 25, 401-402, 1972
- Blumberg B.S, Mc Lartin L,, Lanchat M and Guinto RS. Association between lepromatous leprosy and Australia antigen. Lancet 2: 173- 176, 1967.
- Datta RN, Saha K. Au Antigen in lepromatous leprosy, its incidence and persistence and relation to cell mediated immunity. Ind J Med Res. 61: 1758-1765, 1973.
- Chakrawarti MS,Mukharjee KK, Chakrawarti SK, Ghostis, Chaudhary S. Hepatitis b surface Antigen (HBsAg) in leprosy patients of Calcutta – its prevalence and subtypes. Lepr in India. 51 : 182,1979.
- Kelkar SS, Niphadkar KB, khare PM, Gharpuray MB. Environment and carriage of hepatitis B Ag in leprosy. Ind J Med Res. 62 :1794-1799, 1974.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License