IJCRR - 5(15), August, 2013
Pages: 01-05
Date of Publication: 17-Aug-2013
Print Article
Download XML Download PDF
STUDIES ON THE SUB CULTURE AND MULTIPLICATION OF ANTHURIUMBICOLOUR PLANTLETS IN MURASHIGE SKOOG LIQUID MEDIUM
Author: Ancy D., Bopaiah A.K.
Category: General Sciences
Abstract:This work was carried out to study the multiplication and growth of Anthurium bicolour (Agnihothri). Plantlets obtained earlier in the solid medium. The liquid culture method showed better growth and development of plantlets. The plantlets obtained were bigger in size with respect to leaf area and shoot height. The multiplication rate was enhanced due to faster absorption of nutrients. Rooting was also better in the liquid medium when compared to the solid medium. The study confirmed that liquid MS medium supplemented with 4 mg\L- BA and 1mg\L- NAA is found more suitable for the growth and multiplication of Anthurium bicolor plantlets.
Keywords: Anthurium Bicolor (Agnihothri), In vitro, regeneration, plantlets.
Full Text:
INTRODUCTION
Anthurium andreanum has many hybrids which are cultivated as ornamental and for cut flowers, one among them is Anthurium bicolor variety agnihothri with large brightly coloured spathe. It has a combination of blood red and grass green colour, Hence it is called bicolor. Though Anthuriums are cultivated through regenerative method, the regeneration capacity was found to be less among some of the hybrids. There is great demand for the planting material of Anthurium bicolor variety agnihothri both by the hobbyists and growers. The present work was initiated keeping in mind the demand and faster method for propagation of plantlets. The culture were initiated with solidified agar medium and an attempt was made to grow them using liquid medium supplemented with various concentration of growth additives (Table-1).The response was found to be good in the liquid medium when compared to the solid medium.
MATERIAL AND METHODS
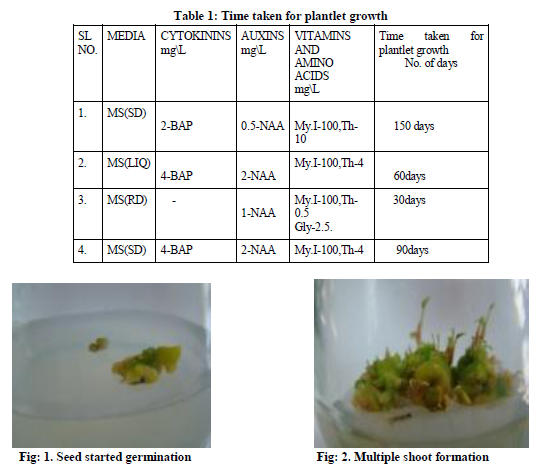
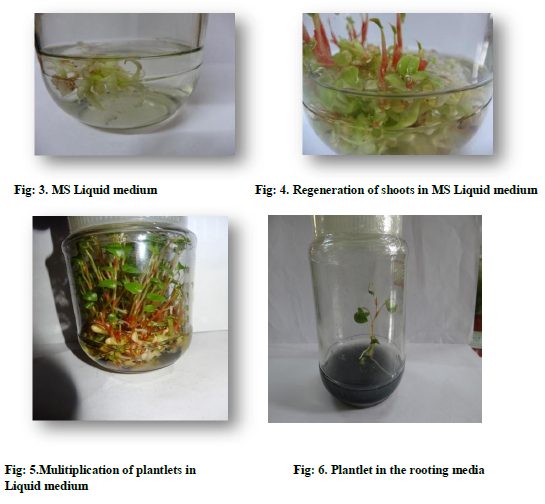
Dry matured seeds were collected from the plant and then wash with running tap water, followed by soaking for 5-8 minutes with a mild liquid detergent (10% v/v) teepol. Again the seed were washed thoroughly with running tap water to remove the detergent trace. Then the seed were surface sterilized by dipping the seed in 0.1% v/v mercuric chloride solution for 10-15 minutes, followed by washing with sterile double distilled water 4 to 5 times inside the laminar air flow. They were again dipped in the hydrogen peroxide for 30 seconds and rinsed with sterile double distilled water, followed the standard procedure .These sterilized seeds were inoculated on the surface of solidified full strength MS (Murashige and Skoog 1962) medium supplement with 2 mg\L-BA and 0.5mg\L-NAA. The seed started germination resulting in multiple shoot formation (fig-2) when the shoots were subcultured in the MS full strength medium supplement with 4 mg\L-BA, 2mg\L-NAA, Myoinositol-100mg\L, thiamine HCl-4mg\L. The plantlets in the solid media was transferred to the liquid medium with same supplements as in the case of solid medium (fig-3). They were incubated at 25oC ±2 oC and (1599-2000 lux) in the growth room. These regenerated shoots formed cluster with bases adhere to one other (fig-5).The liquid medium was not agitated on the rotary shaker as it used to be done in earlier reports (Whei-Lan Teng 1997). Some of the regenerated shoots formed roots at the base, in the liquid medium (fig-7).
The individual shoots were transferred to MS rooting medium (fig-6), supplemented with Myoinositol, thiamine, glycine, NAA, sucrose, 2%-activated charcoal and agar (Table-1), later plantlets transferred to greenhouse.
RESULTS
The seed germinated in full strength MS medium supplemented with 2mg\L and o.5mg\L-NAA, in about 150 days of time the germinated seed showed multiple shoots (fig-2).The plantlets obtained were further subcultured in MS solid medium supplement with 4mg\L-BAP and 2mg\L-NAA. The shoot and roots regenerated in about 60 day of time in the MS liquid medium .Where as in the MS liquid medium, of the same composition as MS solid medium regeneration of plantlets were fast compared to the solid medium. The plantlets was transferred to the root inducing medium supplemented with 1mg\L-NAA, sucrose-3%, activated charcoal-2% (Table-1).
DISCUSSION
It is usual practice to multiply the anthurium plantlets using solidified medium .In the present work an attempt was made to deviate from the monotonous method of culturing them in solidified MS medium. The result was found very positive. The liquid medium was found to be more suitable than the solidified MS medium. This not only saves time in multiplying them. It also saved the cost of producing healthy plantlets, by not using agar in the medium, which is expensive .One more observation made in the is work was usage of, rotary shaker for liquid culture (Whei-Lan Teng 1997). In the present work rotary shaker was totally avoided. The culture were incubated keeping the media in a stagnant condition like the solid media. This also saves the cost of the production by not using extra power for the culture of anthurium plantlets .When the plantlets obtained were transferred to rooting medium (Tables-1), normal root formation was observed (fig-6). When the plantlets were hardened they grew into normal plants without showing any abnormalities (fig-7). This clearly indicates that, by using liquid medium in the later stages of growth, gives better result than the solid medium which is normally used in almost all commercial laboratories.
CONCLUSION
The above work suggest that liquid medium supplement with 4mg\L-BAP and 2mg\L-NAA was found to be better than MS solid media of the same composition for further regeneration and multiplication of plantlets.
ACKNOWLEDGEMENT
The authors wish to thank Dr. Fr. Daniel Fernandes S.J. for is support and encouragement
Financial disclosure: non Funded research work

References:
- Atak C, Celik O. Microprogation of Anthurium andreanum from leaf explants.Pak J Bot (2009); 41(3):115-1161.
- Dufour L. and Guerin V. Growth, developmental features and flower production of Anthurium andreanum Lind. In tropical conditions. Scientia Horticulturae [2003]; vol. 98, no. 1, p. 25-35.
- Dufour L. and Guerin V. Nutrient solution effects on the development and yield of Anthurium andreanum Lind. In tropical soilless conditions.Sci.Horti (2005); 105,269-282.
- Pierik, R.L.M. and Steegmans, H.H.M . Vegetative propagation of Anthurium scherzerianum Schott through callus cultures. Scientia Horticulturae [1976]; vol. 4, p. 291-292.
- Keng Heng Chang, Rung Yi Wu, Keng Change Chuang, Ting Fang Hsieh, Ren Shih Chung. (2010), Effect of chemical and organic fertilizers on the growth, flower quality and nutrient uptake of Anthurium andreanum, Cultivated for cut flower production. Sci.Horti.125, 434-441.
- Mojtaba Khorrami Raad, Sahar Bohluli Zanjani, Mahmoud Shoor, Yousef Hamidoghli, Ali Ramezani Sayyad, Ardashir Kharabian Masouleh and Behzad Kavianni. (2012), Callus induction and organogesis capacity from lamina and petiole explants of Anthurium andreanum Linden (Casino and Antadra).AJCS 6(5):928-937.
- Rout.G.R, Mohapatra.A, Mohan Jain.S. (2006), Tissue culture of ornamental pot plant: A Critical review on present scenario and future prospects. Sci.Horti.24, 531-560.
- Hamidah, M.; Karim, A.G.A. and Debergh, P .Somatic embryogenesis and plant regeneration in Anthurium scherzerianum. Plant Cell Tissue and Organ Culture [1997]; vol. 48, p. 189-193.
- Brunner, I.; Echegaray, A. and Rubluo, A . Isolation and characterization of bacterial contaminant from Dieffenbachia amoena Bull, Anthurium andreanum Linden and Spathiphyllum sp. Shoot cultured invitro. ScientiaHorticulturae [1995]; vol. 62, no. 1-2, p. 103-111.
- Teresa E. Vargas, Alexander Mejia’s, Maria Oropeza, Eva De García. Plant regeneration of Anthurium andreanum CV Rubrun. Electronic Journal of Biotechnology [2004]; ISSN: 0717-3458.
- Budi winarto, Fitri Rachmawat, Dewi Pramanik, Jaime A. Teixeira Da Silva.S. Morphological and cytological diversity of regenerator derived from half –anthers culture of Anthurium. Plant cell tiss organ cult [2011]; 105:363-374
- Cimenatak and Ozgeceelik .Micropropagation of anthurium andreanum from leaf explants.pak.J.Bot [2009]; 41(3):155-161,
- Jagan Mohan Reddy and Bopaiah A.K., Abhilash.M. .In vitro Micropropagation of anthurium digitatum, using leaf as explants. Asian journal of pharmaceutical and health sciences [2011]; vol-1, p.70-74.
- Jagan Mohan Reddy and Bopaiah A.K.studies on the initiation of callusing and regeneration of plantlets in three different basal media with varied plant growth regulators for the micropropagation of anthurium scheraium using leaf an spathe as explants .African J. of biotechnology [2009];vol .11(23).pp.6259-6268.
- Janhan .M.T., Islam M.R., Ruselikhan, Mamum A.N.K., Ahmedd G. and Hakim L.In vitro clonal propagation of Anthurium (Anthurium andreanum L.) using callus culture. [2009];
- Kuehnle AR, Sugii N [1991]. Callus induction and plantlet regeneration in tissue culture of Hawaiian anthurium .Hort.sci [1991]; 26:919-921.
- Martin K.P., Dominic Joseph, Joseph Madassery and V.J.Philip .Direct shoot regeneration from laminal explants of two commercial cut flower cultivars of Anthurium andraeanum Hort.In vitro cell.Dev.BIOL-Plant[2003]; 30:500-504.
- Murashige, T. and Skoog, F .A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiologia Plantarum [2003]; vol. 15, p. 473-497
- Saikat Gantait and Nirmal Mandal .Tissue culture of Anthurium andreanum Significant Review and future Prospective, International journal of Botany [2010]; ISSN 1811-9700
- Whei-Lan Teng, Regeneration of Anthurium adventitious shoots using liquid or raft culture. Plant Cell Tissue and Organ Culture[1997]; vol. 49, no. 2, p. 153-156.
ABBREVIATIONS
6-Benzyl adenine (BAP), α-Napthaleneacetic acid (NAA), Indole-3-acetic acid (IAA), , Murashige and Skoog medium(MS) , Myoinositol(My.I), Glycine(Gly), Thamine (Thy) ,MS Liquid medium(LD),MS Solid medium(SD),MS Rooting medium(RD).
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License