IJCRR - Vol 09 issue 10 current issue , May, 2017
Pages: 11-18
Date of Publication: 27-May-2017
Print Article
Download XML Download PDF
Possibility for Improving Carcass Composition and Meat Quality Traits by Selective Breeding
Author: Trygve Gjedrem
Category: General Sciences
Abstract:An advantage of aquaculture farming compared with fisheries is that it is possible to genetically improve economically important traits through selective breeding. This is possible because many traits have underlying heritable genetic components and individuals carrying those desirable traits can be selected as the parents of the next generation. A difficulty however, is how to measure the quality traits on the potential breeders. Traditionally, measuring the traits on relatives like full- and half-sibs have been applied. However, with new technologies such as near-infrared reflectance spectroscopy, X-ray tomography (CT) and Fotofish, it is now possible to measure fat% and filet color with high precision. Furthermore, new technological advances have opened new doors to potentially estimate fat% and filet color on live animals. Improving fat% by selective breeding is possible, as the heritability of the trait is relatively high (h2 = 0.25) and a genetic gain of 4 % per generation has been reported for rainbow trout. On the other hand, the heritability for flesh color shows high variation, but it is particularly high when measured using CT (h2 = 0.47). Finally, fillet yield has a rather low heritability (h2 = 0.16) and reported genetic gain per generation is close to zero.
Keywords: Aquaculture, Genetic gain, Heritability, Meat quality, Selective breeding
Full Text:
Introduction
Quality traits and carcass composition in fish and shellfish are important characters for both consumers and farmers. In general, from marketing perspective there product can be a complicated process, as different markets may have different quality preferences. For farmers, the size and variation as well as dressing%, fillet yield, shape and color decide the price. Important factors in the market are processors and for salmon smoking operations to improve the product quality. For consumers, the size of the fillet, fat%, texture and color are important quality traits. For Furthermore, in some fish species such as carp and silver barbnumber of intramuscular bones usually have a better market acceptance. This trait seems to be highly variable within species. For instance, it has been reported that the number of intramuscular bones in different ploidies of crucian carp (Carussiusauratus) can vary from 77 to 86[1]. In Blunt snote bream (Megalobramaamblycephala) the number of intermuscular bones is estimated to vary from 108 to 129 [2]For consumers these small bones can be a concern, as they are usually difficult to remove, making them unpopular.
However, one of the main problems of including quality traits in breeding goals is that these traits can not only vary extensively between species, but their preferences can also greatly differ across markets. From a breeding point of view, it is therefore often difficult to decide which traits should be included in the breeding goal and how to rank those traits according to their economic values. With this in mind, the purpose of this short review paper is to discuss possibilities on how to improve quality traits in fish and shellfish through selective breeding. One of the main difficulties of improving carcass and quality traits is how to record the traits on the live animal with high repeatability. However, there are some new promising technologies on the horizon, considering that currently we are mainly relying on recording the traits on the relatives (full- and half-sibs) of potential breeders. Subject scoring has been widely used, which in general has low repeatability and chemical analysis are laborious and expensive. For the purpose of this review, I will not discuss hybridization and monosex culture.
Methods to measure quality traits
In recent years, much work has taken place to investigate different technologies to measure quality traits both on live animals as well as on carcasses. For instance, using ultrasound imaging, Bosworth et al.[3] explained 48-56% and 31-38% of the variation in meat yield traits in female and male catfish, respectively. Sang et al. [4] studied methods to measure quality traits of river catfish by taking measurement of the live fish. The correlation between the predicted and the observed values for fillet weight, fillet yield and fillet fat were 0.93 (5 measurements), 0.86 (4 measurements) and 0.85 (4 measurements). Texture is another example of important quality trait in fresh as well as in processed products. Mørkøre[5] used mechanical pressure to measure texture. It was found that sensory hardness is correlated with a Warner-Blatzler blade of 12.5 mm in diameter in raw salmon (r = 0.70). Gjerde and Martens [7] studied near-infrared reflectance spectroscopy (NIR) to measure water, fat % and protein % in the fillet of rainbow trout. They found very high correlations between chemical analysis and NIR measurements for water, fat % and protein %, ranging from r = 0.97-0.99. Folkestad et al. [6] applied near infrared (VIS/NIR) spectroscopy to estimate fat % in live Atlantic salmon and reported a correlation of 0.94 with chemical estimates. NIR is also an efficient tool for estimating fat % in the gutted and fillet weight. Further, VIS showed to be efficient in estimation of pigments in the fillet. Wold et al. [8] showed that Raman spectroscopy was a suitable method for none destructive estimation of pigments (r = 0.95) and fat % (r = 0.97) in the ground salmon meat.
Computer assisted X-ray tomography (CT) was first used to measure body and carcass composition in live pigs [9]. In rainbow trout, Gjerde [10] estimated correlation between the observed and predicted CT values of 0.88 for water %, 0.89 for fat % and 0.68 for protein %. In a dataset of 174 Atlantic salmon, Rye [11] obtained a correlation of 0.92 between the observed and predicted values for fat and water content. For protein, no significant prediction was recorded. Quillet et al. [12] used Distell Fish Fatmeter in selection for muscle lipid content in rainbow trout. Fillet color is another example of important quality trait particularly in salmonid species. In 2003, researchers (Erland Austreng and Kjell Arne Rørvik) at AKVAFORSK (now Nofima) took the initiative to develop an instrument named Fotofish, which was revised later. In addition to color, Fotofish can also be used to measure the % pigment in the fat. Rørvik et al. [13] report correlations between Fotofish predictions and the following quality traits: pigment (mg/kg), r = 0.94 for salmon and r = 0.97 for rainbow trout, fat %, r = 0.95 for salmon and 0.85 for trout, and color score r = 0.95 for both species. At present, there are several efficient methods for obtaining estimations of the most important quality traits with high precision on both live fish as well as on the carcasses. These are potentially important tools and approaches for further improvements of economically important traits in aquatic species.
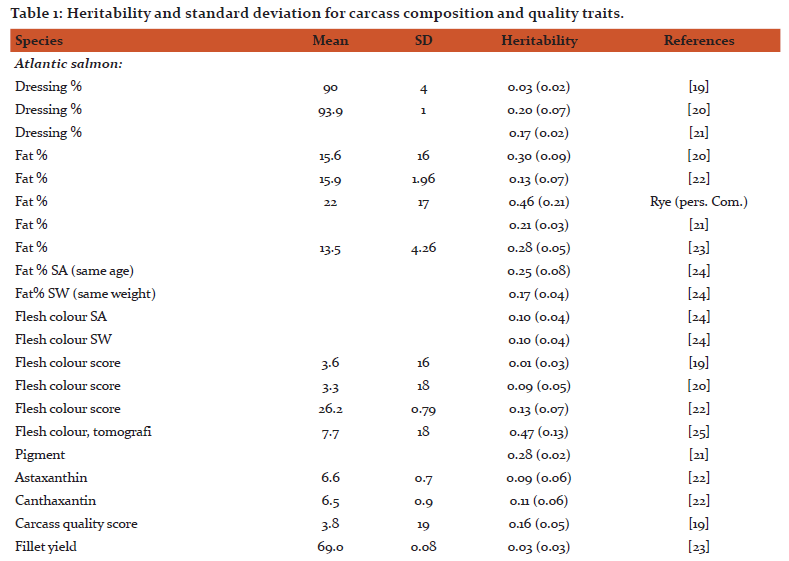
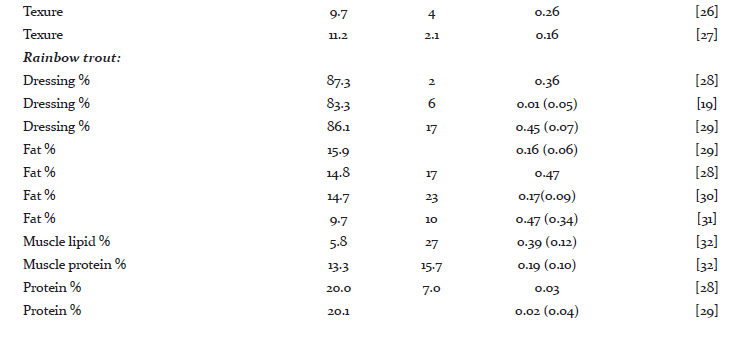
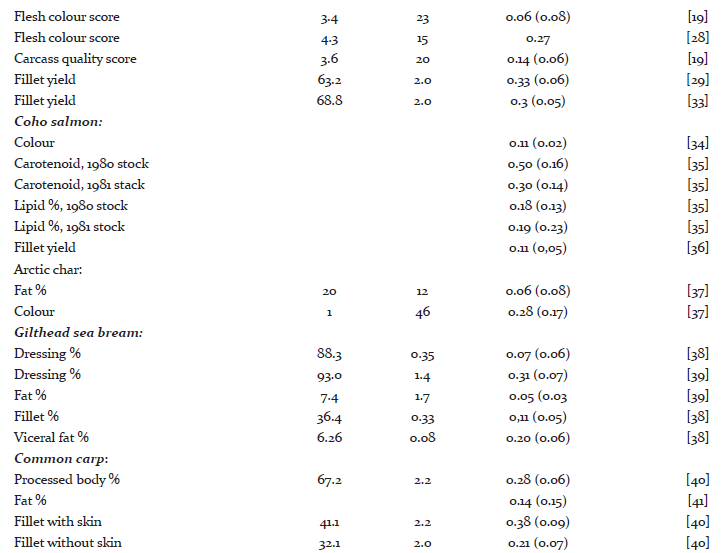
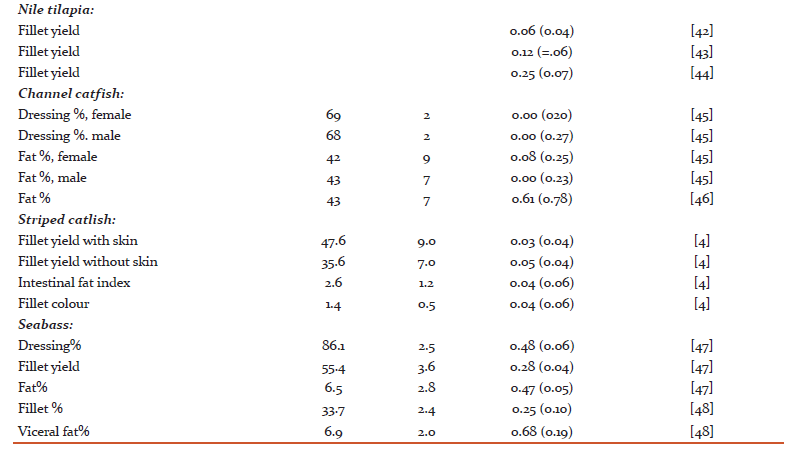
Heritability and genetic variation in quality traits
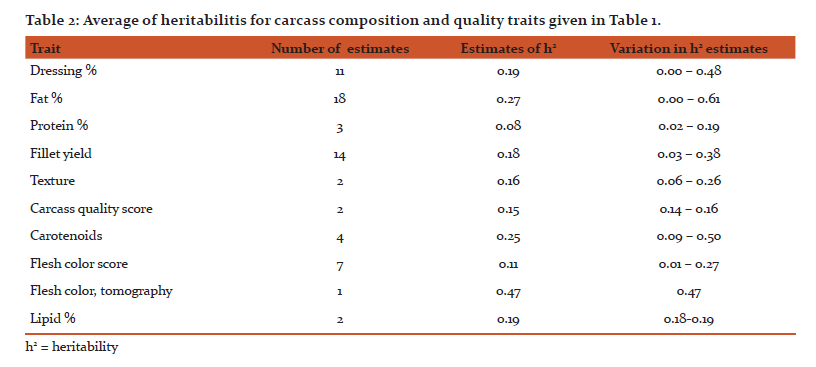
A literature survey for heritability estimates of quality traits and their averages have been presented in Tables 1 and 2, respectively. For most traits, the heritability estimates seem to vary considerably. Such variations might be due to low repeatability of measurements, variations among populations, species and differences in environmental conditions that the animals were exposed to. On average, the reported heritability estimates for protein % is close to zero (h2 = 0.03), the heritability for carcass and quality score are rather low (h2 = 0.10 - 0.15) and this is further followed by h2 = 0.16 for dressing % and fillet texture. For fillet yield there are at least 10 different h2 estimates, with the average across all estimates to be approximately 0.16. The fat % and carotenoids both seem to have a moderate heritability (h2 = 0.25). On the other hand, the heritability for flesh color, measured by tomography, is very high (h2 = 0.47). In general, we can see that most economic traits of interest show genetic variation [14, 15]. For many of these traits extensive work is now underway to better understand their underlying molecular architecture. For example Wan et al. [16] showed that abundant microRNA were functionally involved in regulating the development and differentiation of intermuscular bones and connective tissues in the blunt snout bream. Bai et al. [17]investigated quality traits in triangle pearl mussel. The authors found four QTL explaining 26%-28% of the trait variation, which will provide valuable information for marker-assisted selection of the freshwater pearl mussel. Kuang et al. [18] investigated the genetic variation in fat % in common carp. A genome-wide significant QTL was detected, explaining 36.2% of the phenotypic variance. Further, they identified three QTL with significant effects explaining 14.3%-19.5% of fat content in the common carp. These findings suggest that the observed phenotypic variations of many quality traits might mainly be explained by a few major genes and QTL.
Phenotypic and genetic correlations between quality traits
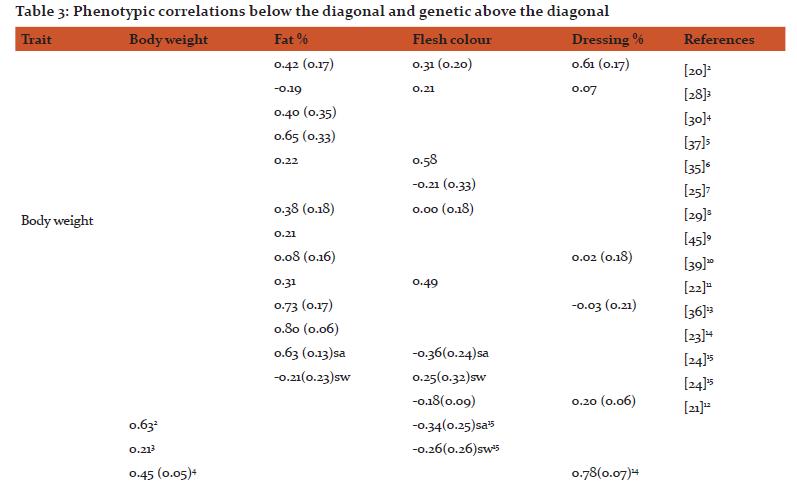
While the phenotypic correlations (rP) between body weight and fat % are positive, varying from 0.05%-0.72%, the genetic correlations (rG) have been reported to range from -0.21 to 0.80 (Table 3). On the other hand, both phenotypic and estimated genetic correlations between body weight and flesh color are low with one exception (rG = -0.21). The genetic correlations between fat % and flesh color are also all low and negative while the phenotypic correlations vary from low negative to high positive. Both phenotypic and genetic correlations between dressing % and quality traits are low with a couple of exceptions (rG = -0.03 with body weight and rP= -0.22 with flesh color). Quality traits are in general measured on carcasses of animals, all with the same age but usually of varying sizes. Using a different approach, Kristiansson [24] estimated correlations between quality traits and body weight on animals of the same age (sa) and similar weights (sw). Both phenotypic and genetic correlations between body weight (sa) and fat % were high and significant. On the other hand, the genetic and phenotypic correlations between weight (sw) and fat % was low. Haffray et al. [33] estimated high negative genetic correlations between body weight and body tissue development as head yield or the head and vertebral column yield (-0.48 to -0.57).
Genetic gain
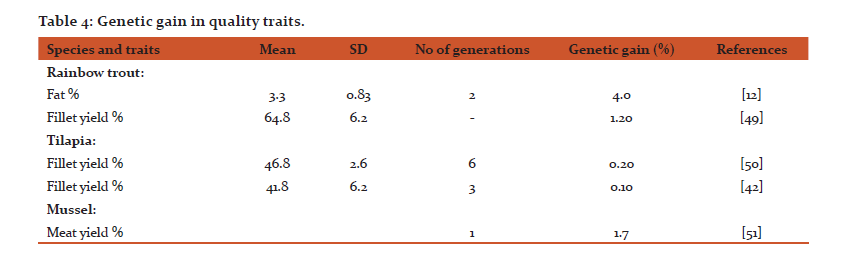
Table 3 shows estimates of genetic gain for some important traits. For fat % in rainbow trout a genetic gain of 4% per generation has been reported [12] . This means that it is possible to improve this important quality trait in farmed fish only over a few generations. For filet yield, the pattern is different. For Nile tilapia, the genetic gain is close to zero as it was estimated for the meat yield in mussel.
Conclusion
Fish and shellfish both from fisheries and aquaculture are healthy food for people, considering their protein and fat contents and important micronutrients such as omega 3 fatty acids. In aquaculture, it is possible to change feed composition and in addition, it is possible to change and improve product quality and carcass composition through selective breeding. For some traits, at least, the possibilities for genetic improvement is highly promising, with perhaps one of the best examples being the fillet fat % which shows moderate heritability (h2 = 0.25) and a genetic gain of 4 % per generation in rainbow trout. Of course, while in some species it might be of interest to increase fat %, in some other species it might be more desirable to reduce it. By using new technological, it is possible to measure various traits like flesh color and carotenoids on the live animal opens numerous possibilities and opportunities for genetic improvements of these economically important traits, particularly in salmonids. For protein % the heritability is close to zero (h2 = 0.03) indicating low genetic variation and no response to selection. For fillet yield the heritability is rather low (h2 = 0.16) and the two estimates of genetic gain in tilapia and one in mussel are close to zero. Gjerde et al. [42] discussed the low genetic gain in tilapia, and concluded: “The close to unity genetic correlation between round body weight and fillet weight indicates that genetic improvement of one of the traits without achieving a proportional genetic change in other is difficult or even impossible. This implies that improvement of fillet yield through direct selection is difficult to achieve”.
Acknowledgment
Author acknowledge the immense help received from the scholars whose articles are cited and included in references of this manuscript.. Are cited and included in references of this manuscript. The author are also grateful to authors/ editors/ publishers of all those articles, journals and books from where the literature for this revive has been reviewed and discussed. I am grateful to Nofima who funded this work. I would also like to thank Dr. Hooman Moghadam for his review and comments on the manuscript,


References:
1. Li, L., Zhong, Z., Zeng, M., Liu, S., Zhou, Y., Xiao, J., Wang, J., Liu, Y. Comparative analysis of intermuscular bones in fish of different ploidies. Science China Life Sciences 2013. 56: 341 – 350.
2. Wan, S., Yi, S.,Zhong. J., Wang, W., Jiang, E., Cheng, B., Gao, Z. Developmental and morphological observation of intermuscular bones in Megalobramaamblycephala. Acta HyrobiologicaSinica, 2014. 38: 1143-1151.
3. Bosworth, B.G., Holland, M., Brazil, B.L. Evaluation of ultrasound imagery and body shape to predict carcass and fillet yield in farm-raised catfish.J. Anim. Sci. 2001. 79: 1483-1490.
4.Sang, N., Thomassen, M., Klemetsdal. H., Gjøen, H.M. Prediction of fillet weight, fillet yield, and fillet fat for live river catfish (Pangasianodonhypophthalmus). Aquaculture 2009. 288: 166-171.
5. Mørkøre, T. Relating sensory and instrumental texture analysis of Atlantic salmon. 2002, Agricultural Collegue of Norway.
6. Gjerde, B., Martens, H. Predicting carcass composition of rainbow trout by near-infrared reflectance spectoscopy. J. Anim. Breed. Genet, 1987. 104: 137-148.
7. Folkestad, A., Wold, J.P., Rørvik, K.J., Tschudi, J., Haugholt, K.H., Kolstad, K., Mørkøre, T. Rapid and non-invasive measurments of fat and pigment concentrations in live and slaughtered Atlantic salmon (Salmo salar L). Aquaculture 2008. 280: 129-135.
8. Wold, J. P., Marquardt, B.K., Dable, B.K., Robb. D., Halen. B. Rapid quantification of carotenoids and fat in Atlantic salmon (Salmo salar L.) by Raman spectroscopy and chemometrics. Applied Spectroscopy, 2004. 58: 395-403.
9. Skjervold, H., Grønseth, K., Vangen, O., Evensen, A. In vivo estimation of body composition by computerized tomography. ZeitschriftfürTierzüchtung und Züchtungsbiologie, 1981. 98(1-4): 77-79.
10. Gjerde, B. Predicting carcass composition of rainbow trout by computerized tomography. Journal of Animal Breeding and Genetics, 1987. 104(1-5): 121-136.
11. Rye, M. Prediction of carcass composition in Atlantic salmon by computerized tomography. Aquaculture, 1991. 99(1): 35-48.
12. Quillet, E., Guillou, S.L., Aubin, J., Fauconneau, B. Two-way selection for muscle lipid content in pan-size rainbow trout (Oncorhyncus mykiss). Aquaculture, 2005. 245 (1-4): 49-61.
13. Rørvik, K., Rørvik, S., Salberg, T., Larsson, T. How red is salmon ? (Hvor rød er laks?). Norsk Fiskeoppdrett, 2014. 11: 54-58.
14. Moav, R., Finkel., A.Wohlfarth, G. Variability of intermuscular bones, vertebrae, ribs, dorsal fin rays and skeletal disorders in the common carp. Theoretical and Applied Genetics, 1975. 46(1): 33-43.
15. Kossman, H. Untersuchungenuber die genetische Variantz der Zwischenmuskelgraten des Karpfens. Theoret. Appl. Geneties 1972. 42: 130-135.
16. Wan, S., Kui, Y., Zhong, J., Nie. C.H., Guan, N.N., Chen, B.X., Gao, Z.X. Identification of microRNA for intramuscular bone development in Blunt Snot Bream (Megalobramaamblycephala). Int. J. Sci, 2015. 16: 10686-10703.
17. Bai, Z., Han, X., Luo, M., Lin, J., Wang, G., Li, J. Constructing a microsatellite-based linkage map and identifying QTL for pearl quality traits in triangle pearl mussel (Hyriopsiscumingii). Aquaculture, 2015. 437: 102-110.
18. Kuang, Y., Zheng, X., Lv, W., Cao, D., Sun, X. Mapping quantitative trait loci for flesh fat content in common carp (Cyprinuscarpio). Aquaculture, 2015. 435: 100-105.
19 Gjerde, B., Gjedrem, T, Estimates of phenotypic and genetic parameters for carcass traits in Atlantic salmon and rainbow trout. Aquaculture, 1984. 36(1–2): 97-110.
20 Rye, M., Gjerde. B. Phenotypic and genetic parameters of composition traits and flesh color in Atlantic salmon. Aquaculture Research 1996. 27: 121-133.
21. Vieira, V., Norri, A., Johnston, I. Heritability of fibre number and size parameters and their genetic relationship to flesh quality traits in Atlantic salmon (Salmo salar L.). Aquaculture 2007. 272: 100-109.
22 Qinton, C.D., McMillan, I., Glebe, B.D. Development of an Atlantic salmon (Salmo salar) genetic improvement program: Genetic parameters of harvest body weight and carcass quality traits estimated with animal models. Aquaculture, 2005. 247(1–4): 211-217.
23 Powell, J., White, I., Guy, D., Brotherstone, S. Genetic parameters of production traits in Atlantic salmon (Salmo salar). Aquaculture, 2008. 274(2–4): 225-231.
24. Kristjánsson, Ó., Gjerde, B., Lillehammer, M., Jonasson, J. Genetic parameters in Atlantic salmon for growth rate and carcass quality traits recorded at the same body weight or the same age. in ISGA XIII. 2015. Santiago de Compostela, Spain.
25. Rye, M., Storebakken, T., Gjerde, B., Ulla, O. Efficient selection for colour in Atlantic salmon (Effektivseleksjon for innfarging hos laks). Norsk Fiskeoppdrett, 1994 11A: 22-24.
26. Refstie, T., Mørkøre, T., Johnsen H., Gjøen, H.M. Better texture in Norwegian salmon through selective breeding. (Bedre textur hos norsk laks gjennom avlsarbeidet). AKVAFORSK - Report 12/99. Texure. 1999. pp19.
27. Larsson, T., Mørkøre, T., Kolstad, K., Østby, T. K., Afanasyev, S., Krasnov, A. Gene expression profiling of soft and firm Atlantic Salmon fillet. PLoS ONE, 2012. 7(6): 1-9, e39219.
28. Gjerde, B., Schaeffer, L.R. Body traits in rainbow trout: II. Estimates of heritabilities and of phenotypic and genetic correlations. Aquaculture, 1989. 80(1–2): 25-44.
29. Kause, A., Ritola, O., Paananen, T., Mantysaari, E., Eskelinen, U. Coupling body weight and its composition: a quantitative genetic analysis in rainbow trout. Aquaculture, 2002. 211(1–4): 65-79.
30. Elvingson, P. Estimation of phenotypic and genetic parameters of carcass lipid content in rainbow trout. 1992, Swedish University of Agricultural Sciences.
31. Kinghorn, B.P. Genetic variation in food conversion efficiency and growth in rainbow trout. Aquaculture. 1983, 32: 141-155.
32. Tobin, D., Kause, A., Mantysaari, E.A., Martin, S.A.M., Houlihan, D.f., Dobly, A., Kiessling, A., Rungruangsak-Torrissen, K., Ritola, O., Ruohonen, K. Fat or lean? The quantitative genetic basis for selection strategies of muscle and body composition traits in breeding schemes of rainbow trout (Oncorhynchus mykiss). Aquaculture 2006. 261: 510-521.
33. Haffray, P., Bugeon, J., Pincent, C., Chapuis, H., Mazeiraud, E., Rossignol, M.N., Chatain, B., Vandeputte,M., Dupont-Nivet, M. Negative genetic correlations between production traits and head or tissues in large all-female rainbow trout (Oncorhynchus mykiss). Aquaculture 2012: 368-369: 145-152.
34. Cerda, C., Neira, R., Barris N. Genetic and phenotypic parameters for harvest weight, color flesh and fat content in coho salmon (Oncorhynchuskisutch). International Association for genetics in Aquaculture, Genetics in aquaculture VIII. 2003. Puerto Varas, Chile.
35. Iwamoto, R,N., Myers, J,M., Hershberger, W., K. Heritability and genetic correlations for flesh coloration in pen-reared coho salmon. Aquaculture, 1990. 86(2): 181-190.
36. Neira, R., Lhorente, J.P.,Raneda, C., Diaz, N., Bustos, E., Alert, A. Studies on carcass quality traits in two populations of Coho salmon (Oncorhynchuskisutch): phenotypic and genetic parameters. Aquaculture, 2004. 241(1–4): 117-131.
37. Elvingson, P., Nilsson, J. Phenotypic and genetic parameters of of body and compositional traits in Arctic char (Salvelinousalpinus) 1992, Swedish University of Agricultural Sciences. 13pp.
38. García-Celdrán, M., Ramis, G., Manchado, M., Estevez, A., Alfonso, J.M., Armero, E. Estimates of heritabilities and genetic correlations of carcass quality traits in a reared gilthead sea bream (Sparusaurata L.) population sourced from three broodstocks along the Spanish coasts. Aquaculture, 2015. 446: 175-180.
39. Navarro, A., Zamorano, M. J., Hildebrandt, S., Gines, R., Aguilera, C., Alfonso, J.M. Estimates of heritabilities and genetic correlations for body composition traits and GxE interactions, in gilthead seabream (Sparusauratus L.). Aquaculture 2009. 295: 183-187.
40. Kocour, M., Mauger, S., Rodina, M., Gela, D., Linhart, O., Vandeputte, M. Heritability estimates for processing and quality traits in common carp (Cyprinuscarpio L.) using a molecular pedigree. Aquaculture, 2007. 270(1–4): 43-50.
41. Smisek, J., Considerations of body conformation, heritability and biochemical characters in genetic studies of carp in Czechoslovakia. Buletin VURH Vodnany, 1979. 15: 3-6.
42. Gjerde, B., Mengistu, S.B., Ødegård, J., Johanson, H., Altamirano, H. Quantitative genetics of body weight, fillet weight and fillet yield in Nile tilapia (Oreochromis niloticus). Aquaculture, 2012, 342-343: 117-124.
43. Rutten, M.J.M., Bovenhuis, H., Komen, H. Genetic parameters for fillet traits and body measurements in Nile tilapia (Oreochromis niloticus L.). Aquaculture, 2005. 246(1–4): 125-132.
44. Nguyen, N.H., Ponzoni, R.W., Abu-Bakar., Hamzah, A., Lhaw, H.L., Yee, H.Y. Correlated response in fillet weight and yield to selection for increased harvest weight in genetically improved farmed tilapia (GIFT strain), Oreochromis niloticus. Aquaculture, 2010. 305(1–4): 1-5.
45.El-Ibiary, H., Joyce, J. Heritability for body traits, dressing weight and lipid content in channel catfish. Journal of Animal science, 1978. 47: 82-88.
46. Reagan Jr, R.E. Heritability and genetic correlations of desirable commercial traits in channel catfish. 1980, USA: Compl. Rep. Miss. Agric For. Exp. Stn. Publ. by Mafes-MS.
47.Vandeputte. M., Garouste, R., Dupont-Nivet, M., Hsaffray, P., Vergnet, A., Chavanne, H., Laureau, S., Ron, T.B., Pagelson, G., Mazorra, C., Ricoux, R., Marques, P., Gameiro, M., Chatain, B. Multi-site evaluation of the rearing performances of 5 wild populations of European sea bass (Dicentrarchuslabrax). Aquaculture, 2014: 424-425: 230-248.
48. Saillant, E., Dupont-Nivet, M., Sabourault, M., Haffray, P., Laureau, S., Vidal, M.O., Chatain, B. Genetic variation for carcass quality traits in cultured sea bass (Dicentrarchuslabrax). Aquatic Living Resources, 2009: 22: 105-112.
49. Kause, A., Paananen, T., Ritola, O., Koskinen, H. Direct and indirect selection of visceral lipid weight, fillet weight, and fillet percentage in rainbow trout breeding program. J Anim Sci, 2007. 85(12): 3218-27.
50. Thodesen, J. (Ma, D,Y.), Rye, M., Wang, Y.X., Li, S.J., Bentsen, H.B., Gjedrem, T. Genetic improvement of tilapia in China: Genetic parameters and selection responses in filet traits of Nile tilapia (Oreochromis niloticus) after six generations of multi-trait selection for growth and fillet yield. Aquaculture, 2012: 366-367: 67-75.
51. Nguyen, TTT., Hayes, B., Ingram, B.A. Genetic parameters and response to selection in blue mussel (Mytiluscalloprovincialis) using a SNP base pedigree. Aquaculture, 2014: 420-421: 295-301.
|






 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License